Natural Products
Quercetin-3-O-glucuronide
| Catalog No. | CFN92172 | 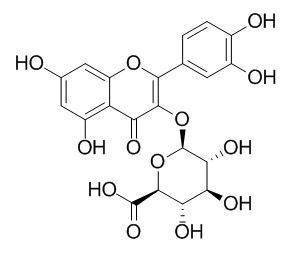 |
| CAS No. | 22688-79-5 | |
| Molecular Weight: | 478.4 | |
| Molecular Formula | C21H18O13 | |
| DBs | [PubChem]:274954220 [ChEMBL]:66395 [PCIDB]:5376 |
Standard InChI:
InChI=1S/C21H18O13/c22-7-4-10(25)12-11(5-7)32-17(6-1-2-8(23)9(24)3-6)18(13(12)26)33-21-16(29)14(27)15(28)19(34-21)20(30)31/h1-5,14-16,19,21-25,27-29H,(H,30,31)/t14?,15-,16?,19-,21+/m0/s1
Biological Activity
Quercetin-3-O-glucuronide, significantly reduces the generation of β-amyloid (Aβ) peptides by primary neuron cultures generated from the Tg2576 AD mouse model, brain-targeted quercetin-3-O-glucuronide may simultaneously modulate multiple independent AD disease-modifying mechanisms , thus, it may contribute to the benefits of dietary supplementation with red wines as an effective intervention for AD.[1]
Quercetin-3-O-glucuronide (0.1μM) suppresses invasion of MDA-MB-231 breast cancer cells and MMP-9 induction, and inhibited the binding of [ 3 H]-NA to β 2 -AR, suggests that it may function to suppress invasion of breast cancer cells by controlling β 2 -adrenergic signaling, and may be a dietary chemopreventive factor for stress-related breast cancer.[2]
Quercetin-3-O-glucuronide are equally effective in inhibiting ROS-associated inflammation and ameliorating insulin resistant endothelial dysfunction by beneficial regulation of IRS-1 function.[3]
Quercetin-3-O-glucuronide is a potential anti-atherogenic metabolite, enhancing the anti-inflammatory properties of M2a macrophages and modulating effects in the presence of pro-inflammatory stimuli.[4]
Quercetin-3-O-glucuronide has anti-neuroinflammatory effects on LPS-induced neuroinflammation in BV2 Cells.[5]
Quercetin-3-O-glucuronide induces ABCA1 in macrophages, and to provide an alternative explanation to previous studies on arteriosclerosis prevention by quercetin.[6]
Product
Official website: Quercetin-3-O-glucuronide
Japanese website: Quercetin-3-O-glucuronide
Chinese website: Quercetin-3-O-glucuronide
Japanese website: Quercetin-3-O-glucuronide
Chinese website: Quercetin-3-O-glucuronide
References
[1] Ho L, Ferruzzi M G, Janle E M, et al. 2013, 27(2):769-81.
[2] Yamazaki S, Miyoshi N, Kawabata K, et al. Arch Biochem Biophy, 2014, 557:18-27.
[3] Guo X D, Zhang D Y, Gao X J, et al. Mol Nutr Food Res, 2013, 57(6):1037–45.
[4] Derlindati E, Dall Asta M, Ardigò D, et al. Food & Function, 2012, 3(11):1144-52.
[5] Yoon C S, Kim D C, Ko W M, et al. Korean J Pharma, 2014, 45(1):17-22.
[6] Kazuaki Ohara, Hideyuki Wakabayashi, Yoshimasa Taniguchi, et al. Biochem Bioph Res Co, 2013, 441(4):929-34.
[7] FAN Dong-sheng, ZHAO Chao, CHEN Hua-guo, et al. Journal of Instrumental Analysis, 2012.
Product Use Citation





