Natural Products
Tanshinone IIA
| Catalog No. | CFN98952 | 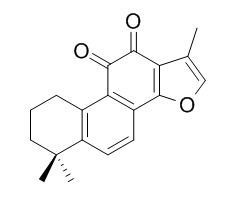 |
| CAS No. | 568-72-9 | |
| Molecular Weight: | 294.4 | |
| Molecular Formula | C19H18O3 | |
| DBs | [PubChem]:274951553 [ChEMBL]: [PCIDB]:31402 |
Standard InChI:
InChI=1S/C19H18O3/c1-10-9-22-18-12-6-7-13-11(5-4-8-19(13,2)3)15(12)17(21)16(20)14(10)18/h6-7,9H,4-5,8H2,1-3H3
Biological Activity
Tanshinone Ⅱ-A is a derivative of phenanthrenequinone isolated from Salvia miltiorrhiza BUNGE, a traditional herbal medicine that is known to induce anti-inflammatory, anti-oxidative and cytotoxic activity; tanshione Ⅱ-A induces HL60 and K562 cellular apoptosis that may be associated with the selective members of caspase family[1]
Acetyltanshinone IIA (ATA), a novel anti-cancer agent, preferentially inhibits cell growth of oestrogen receptor positive (ER+) breast cancer cells and that it is more potent than the commonly used anti-breast cancer agent, tamoxifen; ATA also reduces the mRNA levels of the ERα encoding gene, ESR1, and reduces the transcription of an ER-responsive gene, GREB1.[2]
Tanshinone IIA may inhibit LPS-induced IκBα degradation and NF-κB activation via suppression of the NIK–IKK pathway as well as the MAPKs (p38, ERK1/2, and JNK) pathway in RAW 264.7 cells and these properties may provide a potential mechanism that explains the anti-inflammatory activity of tanshinone IIA.[3]
Tanshinone IIA reduces apoptosis induced by hydrogen peroxide in the human endothelium-derived EA.hy926 cells.[4]
Tanshinone IIA has significant growth inhibition effects on THP-1 cells by induction of apoptosis, and that tanshinone IIA-induced apoptosis on THP-1 cells is mainly related to the disruption of Deltapsim and activation of caspase-3 as well as down-regulation of anti-apoptotic protein Bcl-2, survivin and up-regulation of pro-apoptotic protein Bax, suggests Tanshinone IIA may serve as a potential anti-leukemia reagent.[5]
Product
References
[1] Sung H J, Choi S M, Yoon Y, et al. Experimental & Molecular Medicine, 1999, 31(4):174-8.
[2] Yu T, Zhou Z, Mu Y, et al. Cancer Lett, 2014, 346(1):94-103.
[3] Jang S I, Kim H J, Kim Y J, et al. Eur J Pharmacol, 2006, 542(1-3):1-7.
[4] Jia L Q, Yang G L, Ren L, et al. J Ethnopharmacol, 2012, 143(1):100-8.
[5] Liu J J, Zhang Y, Lin D J, et al. Oncol Reports, 2009, 21(4):1075-81.
[6] Lei M U, Chen G, Zhang X, et al. Lishizhen Medicine & Materia Medica Research, 2011, 22(10):2338-41.
Product Use Citation





