Natural Products
Salvianolic acid B
| Catalog No. | CFN99332 | 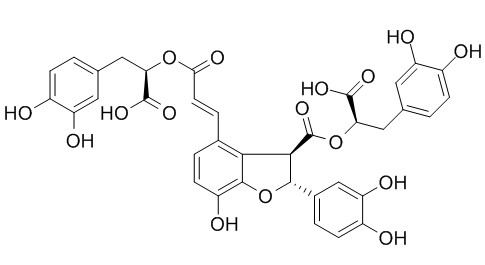 |
| CAS No. | 115939-25-8 | |
| Molecular Weight: | 718.62 | |
| Molecular Formula | C36H30O16 | |
| DBs | [PubChem]:274950818 [ChEMBL]: [PCIDB]:31982 |
Standard InChI:
InChI=1S/C36H30O16/c37-20-6-1-16(11-24(20)41)13-27(34(45)46)50-29(44)10-5-18-3-9-23(40)33-30(18)31(32(52-33)19-4-8-22(39)26(43)15-19)36(49)51-28(35(47)48)14-17-2-7-21(38)25(42)12-17/h1-12,15,27-28,31-32,37-43H,13-14H2,(H,45,46)(H,47,48)/b10-5+/t27-,28-,31+,32-/m1/s1
Biological Activity
Salvianolic acid B (Sal-B) is a bioactive compound isolated from the Chinese medicinal herb Danshen, which is used for treating neoplastic and chronic inflammatory diseases in China, Sal-B inhibits COX-2 expression in cultured HNSCC cells and in HNSCC cells isolated from tumor xenografts, shows as a COX-2 targeted anticancer agent for HNSCC prevention and treatment.[1]
Salvianolic acid B inhibits Abeta fibril formation and disaggregates preformed fibrils and protects against Abeta-induced cytotoxicty, inhibition of Abeta fibril aggregation as one possible method to halt the progression of Alzheimer's disease (AD), so salvianolic acid B has therapeutic potential in the treatment of AD.[2]
Salvianolic acid B exerts neuroprotective effects against H 2 O 2 toxicity, which might be of importance and contribute to its clinical efficacy for the treatment of neurodegenerative diseases. [3]
Salvianolic acid B has antioxidative potential, can reduce the 6-hydroxydopamine-induced increase of caspase-3 activity, and reduce C translocation into the from mitochondria, may be effective in treating associated with oxidative stress.[4]
Salvianolic acid B exerts various anti-oxidative and anti-inflammatory activities in in vitro and in vivo studies, SalB (25 mg/kg) can reduce brain edema, lesion volume and motor functional deficits, and improve spatial learning and memory abilities.[5]
Product
Official website: Salvianolic acid B
Japanese website: Salvianolic acid B
Chinese website: Salvianolic acid B
Japanese website: Salvianolic acid B
Chinese website: Salvianolic acid B
References
[1] Hao Y, Xie T, Korotcov A, et al. Int J Cancer, 2009, 124(9):2200-9.
[2] Durairajan SS, Yuan Q, Xie L,et al. Chan WSNeurochemistry International, 2008, 52(4-5):741-50.
[3] Liu C, Chen N J.Phytomedicine, 2007, 14(7-8):492-7.
[4] Tian L L, Wang X J, Sun Y N, et al. Int J Biochem Cell B, 2008, 40(3):409-22.
[5] Dong J, Liu Y, Liang Z, et al. Ultrason Sonochem, 2010, 17(1):61-5.
[6] Gao J F, Ding L, Zhang P, et al. China Pharmacy, 2014.
Product Use Citation





