Natural Products
Conophylline
| Catalog No. | CFN99473 | 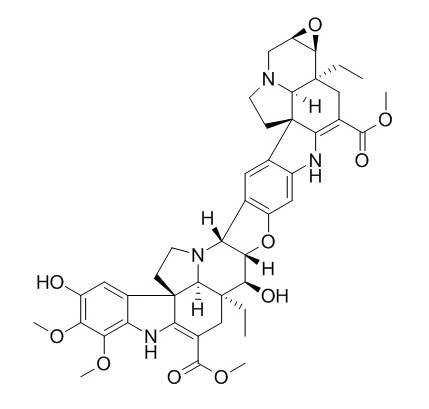 |
| CAS No. | 142741-24-0 | |
| Molecular Weight: | 794.9 | |
| Molecular Formula | C44H50N4O10 | |
| DBs | [PubChem]:274950679 [ChEMBL]: [PCIDB]: |
Standard InChI:
InChI=1S/C44H50N4O10/c1-7-41-16-20(37(51)55-5)34-44(23-14-25(49)30(53-3)31(54-4)28(23)46-34)10-12-48(40(41)44)29-19-13-22-24(15-26(19)57-32(29)35(41)50)45-33-21(38(52)56-6)17-42(8-2)36-27(58-36)18-47-11-9-43(22,33)39(42)47/h13-15,27,29,32,35-36,39-40,45-46,49-50H,7-12,16-18H2,1-6H3/t27-,29-,32+,35-,36-,39+,40+,41-,42-,43+,44+/m1/s1
Biological Activity
Conophylline, is a bis (indole) alkaloid consisting of two pentacyclic aspidosperma skeletons, plays a key role in the regulation of cell mass proliferation, maintenance of the undifferentiated state of iPMSCs and also promotes iPMSCs differentiated into insulin-producing cells.[1]
Conophylline down-regulates the expression of the TNF-α receptors on the cell surface.
Conophylline is a novel differentiation inducer for pancreatic β cells, can increase the numbers of ductal cells positive for pancreatic-duodenal-homeobox protein-1 and islet-like cell clusters. [2,3]
Conophylline has been shown to induce differentiation of pancreatic AR42J cells and increases the formation of beta-cells.[4]
Conophylline suppresses pancreatic stellate cells and improves islet fibrosis in Goto-Kakizaki rats.[5]
Conophylline decreases the fasting blood glucose level inGoto-Kakizaki rats in a dose-dependent manner after repetitive administration for 42 days, suggests that the extract from conophylline-containing leaves may be useful as a functional food for the treatment of type 2 diabetes mellitus.[6]
Conophylline protects cells in cellular models of neurodegenerative diseases by inducing mTOR-independent autophagy.[7]
Conophylline suppresses hepatic stellate cells and attenuates thioacetamide-induced liver fibrosis in rats.[8]
Product
References
[1] Zhang H R, Dan L I, Hui C, et al. J Integr Agr, 2013, 12(4):678-86.
[2] Gohda, Inoue, Umezawa. Intl J Oncoly, 2003, 23(5):1373-9.
[3] Kojima I, Umezawa K. Int J Biochem Cell Biol, 2006, 38(5-6):923-30.
[4] Ogata T, Li L, Yamada S, et al. Diabetes, 2004, 53(10):2596-602.
[5] Saito R, Yamada S, Yamamoto Y, et al. Endocrinology, 2012, 153(2):621-30.
[6] M Fujii,I Takei,K Umezawa. Biomed Pharmacother, 2009, 63(10):710-716.
[7] Sasazawa Y, Sato N, Umezawa K, et al. J Biol Chem, 2015,1(10):290.
[8] Kubo N, Saito R, Hamano K, et al. Liver Int Official J Intl Associat Study Liver, 2014, 34(7):1057–67.
Product Use Citation





