| Description: |
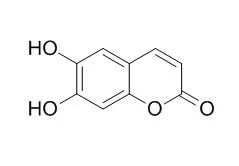
6,7-Dihydroxycoumarin(Esculetin) has various biological and pharmaceutical properties including anti-edema, anti-inflammatory, anti-tumour, hepatoprotective, anti-osteoarthritis and anti-rheumatoid arthritis effects. It inhibits lipoxygenases (LOs), p42/44 MAPK activation, PI3-kinase activation, as well as NF-kappaB and AP-1 activation, it exhibits competitive inhibition against the oxidation of 3-(3,4-dihydroxyphenyl)- alanine by mushroom, the IC50 value of is 43 microM. |
| In vitro: |
| Int J Oncol. 2015 Jan;46(1):265-71. | | Esculetin (6,7-dihydroxycoumarin): a potential cancer chemopreventive agent through suppression of Sp1 in oral squamous cancer cells.[Pubmed: 25310400] | Esculetin (6,7-Dihydroxycoumarin), a coumarin compound, is known to inhibit proliferation and induce apoptosis in several types of human cancer cells and is regarded as a promising chemotherapeutic agent.
METHODS AND RESULTS:
The purpose of the present study was to investigate the anti-proliferative effects of esculetin on two oral squamous cell carcinoma (OSCC) cell lines, HN22 and HSC4, through regulation of specificity protein 1 (Sp1). We examined the apoptotic effects of esculetin were measured by MTS assay, DAPI staining, Annexin V, PI staining, RT-PCR, western blot analysis and immunocytochemistry in HN22 and HSC4 cells. Taken together, the results of the present study indicate that esculetin had anti-proliferative effect on the growth of OSCC cells (HN22 and HSC4) in a dose- and time-dependent manner. The treatment of HN22 and HSC4 cells with esculetin led to a significant reduction in growth and induced apoptosis, followed by the regulation of Sp1 and Sp1 regulatory protein.
CONCLUSIONS:
This indicates that esculetin inhibited cell growth and induced apoptosis by suppressing Sp1 in HN22 and HSC4 cells, suggesting it to be a potent anticancer drug candidate for oral cancer. | | Food Funct. 2014 Sep;5(9):2371-7. | | Esculetin inhibits the inflammatory response by inducing heme oxygenase-1 in cocultured macrophages and adipocytes.[Pubmed: 25088305] |
METHODS AND RESULTS:
In this study, we investigated the anti-inflammatory effects of esculetin (6,7-Dihydroxycoumarin,ECT) through up-regulation of heme oxygenase-1 (HO-1) in cocultured macrophages and adipocytes. RAW264.7 macrophages and differentiated 3T3-L1 adipocytes were cocultured in serum-free Dulbecco's modified Eagle's medium with or without ECT for 24 h. Nitric oxide (NO), tumor necrosis factor-α (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) production was measured in the coculture supernatant. ECT decreased the secretion of NO, TNF-α, and MCP-1. The expression of adipogenic proteins, including peroxisome proliferator-activated receptors γ (PPARγ) and CCAAT/enhancer binding protein α (C/EBPα) in cocultured adipocytes and inducible nitric oxide synthase (iNOS) in cocultured macrophages, was inhibited by ECT. Additionally, HO-1 expression was induced in cocultured macrophages and adipocytes. Silencing of HO-1 expression increased the production of NO, TNF-α, and MCP-1 in cocultured cells, in spite of the presence of ECT.
CONCLUSIONS:
This study demonstrated that ECT exhibited anti-inflammatory properties by inhibiting the production of proinflammatory cytokines in the interaction between adipocytes and macrophages through HO-1 expression. ECT may have the potential to improve chronic inflammation in obesity. | | Eur. J. Pharmacol., 1999, 370(3):297-305 | | Esculetin suppresses proteoglycan metabolism by inhibiting the production of matrix metalloproteinases in rabbit chondrocytes.[Pubmed: 10334506] | The possible mechanism of the chondroprotective effect of 6,7-Dihydroxycoumarin (esculetin) was investigated using primary cultures of rabbit articular chondrocytes.
METHODS AND RESULTS:
Esculetin (EST) significantly suppressed the proteoglycan depletion and the release of pulse-labeled [35S]proteoglycan from the matrix layer of rabbit chondrocytes treated with recombinant human interleukin-1alpha. The matrix metalloproteinase inhibitor, 1,10-phenanthroline, also blocked the proteoglycan depletion and [35S]proteoglycan release. From these results, it is likely that recombinant human interleukin-1alpha-induced proteoglycan depletion is mediated by matrix metalloproteinases. Although esculetin did not directly inhibit collagenolytic activity in the culture media, it significantly suppressed the production of pro-matrix metalloproteinase-1/interstitial procollagenase and pro-matrix metalloproteinase-3/prostromelysin 1, accompanied by a decrease in the steady-state levels of their mRNAs.
CONCLUSIONS:
These results suggest that esculetin is a therapeutically effective candidate for inhibition of cartilage destruction in osteoarthritis and rheumatoid arthritis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)