| In vitro: |
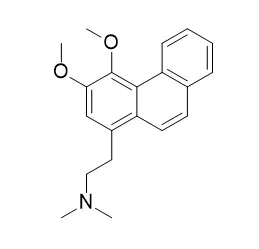
| Molecules. 2013 Jul 8;18(7):8009-17. | | Antiplasmodial alkaloids from the bark of Cryptocarya nigra (Lauraceae).[Pubmed: 23884132] |
METHODS AND RESULTS:
A dichloromethane extract of the stem bark of Cryptocarya nigra showed strong in vitro inhibition of Plasmodium falciparum growth, with an IC50 value of 2.82 μg/mL. The phytochemical study of this extract has led to the isolation and characterization of four known alkaloids: (+)-N-methylisococlaurine (1), Atherosperminine (2), 2-hydroxyathersperminine (3), and norAtherosperminine (4). Structural elucidation of all alkaloids was accomplished by means of high field 1D- and 2D-NMR, IR, UV and LCMS spectral data. The isolated extract constituents (+)-N-methylisococlaurine (1), Atherosperminine (2) and 2-hydroxy-Atherosperminine (3) showed strong antiplasmodial activity, with IC50 values of 5.40, 5.80 and 0.75 μM, respectively. In addition, (+)-N-methylisocolaurine (1) and Atherosperminine (2) showed high antioxidant activity in a DPPH assay with IC50 values of 29.56 ug/mL and 54.53 ug/mL respectively.
CONCLUSIONS:
Compounds 1 and 2 also both showed high antioxidant activity in the FRAP assay, with percentages of 78.54 and 70.66 respectively and in the metal chelating assay, with IC50 values of 50.08 ug/mL and 42.87 ug/mL, respectively. | | Eur J Pharmacol. 1993 Jun 11;237(1):109-16. | | The relaxant actions on guinea-pig trachealis of atherosperminine isolated from Fissistigma glaucescens.[Pubmed: 8395388] |
The pharmacological activity of Atherosperminine, isolated from Fissistigma glaucescens, was determined in isolated guinea-pig trachealis.
METHODS AND RESULTS:
Atherosperminine (25-100 microM) and theophylline (10-1000 microM) both inhibited the contractile response caused by carbachol, prostaglandin F2 alpha (PGF2 alpha), U46619 (thromboxane A2 analogue), leukotriene C4 (LTC4) and Ca2+ (in the presence of 120 mM KCl) in a concentration-dependent manner. The inhibition was characterized by a rightwards shift of the concentration-response curves with suppression of the maximal contraction. Propranolol (1 microM), glibenclamide (10 microM) and removal of tracheal epithelium did not modify the relaxant action of Atherosperminine. Atherosperminine (25 and 50 microM) caused a 2.4- and 5.0-fold, respectively, potentiation of the action of forskolin to cause tracheal relaxation but did not potentiate the action of sodium nitroprusside or cromakalim. Atherosperminine (50 microM) potentiated the action of forskolin to increase tissue cAMP content and, in higher concentrations (100 and 250 microM), itself increased tissue cAMP but not cGMP content. Atherosperminine markedly inhibited cAMP phosphodiesterase but not cGMP phosphodiesterase in homogenates of guinea-pig trachealis.
CONCLUSIONS:
It is concluded that Atherosperminine exerts a non-specific relaxant effect on the trachealis. Its major mechanism of action appears to be inhibition of cAMP phosphodiesterase, perhaps with a minor effect on cGMP phosphodiesterase at higher concentrations. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)