| Structure Identification: |
| Nat Prod Res. 2013;27(1):80-4. | | Steroids and phenolic constituents from the fruiting bodies of the basidiomycete Sarcodon joedes.[Pubmed: 22320163] | Nine secondary metabolites, including four steroids, four phenolics and one cerebroside, were isolated from the methanol extract of the fruiting bodies of the basidiomycete Sarcodon joedes.
METHODS AND RESULTS:
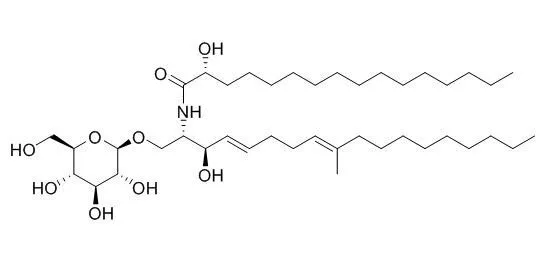
The isolated compounds were identified by spectroscopic analyses as (22E,24R)-6β-methoxyergosta-7,22-diene-3β,5α-diol (1), 2',3'-diacetoxy-3,4,5',6',4″-pentahydroxy-p-terphenyl (2), Cerebroside B (3), ergosta-7,22-dien-3β-ol (4), ergosterol peroxide (5), (22E,24R)-3β-hydroxy-ergosta-5,22-dien-7-one (6), benzoic acid (7), methyl p-hydroxybenzoate (8) and 3,4-dihydroxybenzoic acid (9). The cytotoxic activities of these compounds were evaluated.
CONCLUSIONS:
All these compounds were isolated from this fungus for the first time. | | Tetrahedron, 1989, 45(23):7263-80. | | Synthesis of cerebroside B1b with antiulcerogenic activity I. Synthesis of ceramides with optically active α-hydroxypalmitic acids.[Reference: WebLink] |
METHODS AND RESULTS:
Synthesis of Cerebroside B1b with antiulcerogenic activity I. Synthesis of ceramides with optically active α-hydroxypalmitic acids. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)