| Kinase Assay: |
| Biol Pharm Bull. 2006 Aug;29(8):1639-44. | | The course of uncarinic acid E-induced apoptosis of HepG2 cells from damage to DNA and p53 activation to mitochondrial release of cytochrome c.[Pubmed: 16880619] | Uncarinic acid E, an active component isolated from Gelsemium elegans BENTH, has been reported to exhibit antitumor effects, but little is known about its molecular mechanisms of action.
METHODS AND RESULTS:
In this study, the growth-inhibitory activity of Uncarinic acid E for HepG2 cells is in time- and dose-dependent manner. HepG2 cells treated with Uncarinic acid E exhibited several typical characteristics of apoptosis through photomicroscopical observation, DNA agarose gel electrophoresis. The inhibitory effect of Uncarinic acid E on HepG2 cells was partially reversed by the inhibitors of pan-caspase, caspase-3 and caspase-6. The protein expression ratio of Bcl-xL/Bax and Bcl-2/Bax was down-regulated and Uncarinic acid E-induced apoptosis involves the initial phase mediated by the balance among Bcl-xL, Bcl-2 and Bax proteins, resulting in cytochrome c release from the mitochondria. Uncarinic acid E significantly increased the expression of p53 proteins indicates that p53 plays a pivotal role in the initiation phase of Uncarinic acid E-induced HepG2 cell apoptosis. The phoshatidylinositol 3-kinase (PI3-K) family inhibitor wortmanin and the MEK inhibitor (PD98059) rescued the viability loss induced by Uncarinic acid E through the expression of p53.
CONCLUSIONS:
Taken together, Uncarinic acid E induces apoptosis in HepG2 cells via accumulation of p53, alters the Bax/Bcl-2 ratio, and activates caspases, resulting in cytochrome c release from the mitochondria. |
|
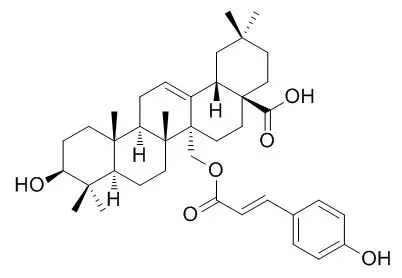
| Structure Identification: |
| Chem Biodivers. 2017 Apr;14(4). | | Cytotoxic Triterpenoids from the Barks of Betula platyphylla var. japonica.[Pubmed: 28052515 ] |
METHODS AND RESULTS:
Phytochemical investigation on the barks of Betula platyphylla var. japonica (Betulaceae) was carried out, resulting in the isolation and identification of three new triterpenoids, 27-O-cis-caffeoylcylicodiscic acid (1), 27-O-cis-feruloylcylicodiscic acid (2), and 27-O-cis-caffeoylmyricerol (3), along with six known triterpenoids, obtusilinin (4), winchic acid (5), 27-O-trans-caffeoylcylicodiscic acid (6), Uncarinic acid E (7), myriceric acid B (8), and 3-O-trans-caffeoyloleanolic acid (9). The structures of the new compounds were elucidated by extensive spectroscopic methods, including 1D- and 2D-NMR, and HR-ESI-MS. All of the isolated compounds were evaluated for cytotoxicity against four human tumor cell lines (A549, SK-OV-3, SK-MEL-2, and Bt549).
CONCLUSIONS:
Compounds 2, 6, 8, and 9 exhibited potent cytotoxicity against all of the tumor cells tested (IC50 < 10.0 μm), while compounds 3, 4, 5, and 7 showed moderate cytotoxicity against all of the tumor cells tested (IC50 < 20.0 μm). |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)