Natural Products
Tannic acid
| Catalog No. | CFN90501 | 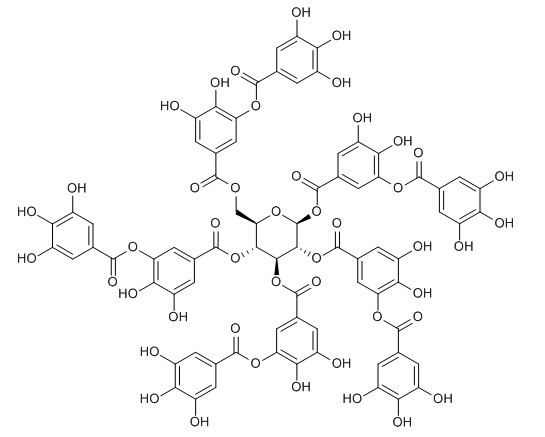 |
| CAS No. | 1401-55-4 | |
| Molecular Weight: | 1701.2 | |
| Molecular Formula | C76H52O46 | |
| DBs | [PubChem]:274954720 [ChEMBL]:75211 [PCIDB]: |
Standard InChI:
InChI=1S/C76H52O46/c77-32-1-22(2-33(78)53(32)92)67(103)113-47-16-27(11-42(87)58(47)97)66(102)112-21-52-63(119-72(108)28-12-43(88)59(98)48(17-28)114-68(104)23-3-34(79)54(93)35(80)4-23)64(120-73(109)29-13-44(89)60(99)49(18-29)115-69(105)24-5-36(81)55(94)37(82)6-24)65(121-74(110)30-14-45(90)61(100)50(19-30)116-70(106)25-7-38(83)56(95)39(84)8-25)76(118-52)122-75(111)31-15-46(91)62(101)51(20-31)117-71(107)26-9-40(85)57(96)41(86)10-26/h1-20,52,63-65,76-101H,21H2/t52-,63-,64+,65-,76?/m1/s1
Biological Activity
Tannic acid (TA) is a naturally occurring polyphenolic compound with antioxidant and radical scavenging properties as well as anticarcinogenic effects, TA can inhibit the growth inhibitory effects on malignant human cholangiocytes in vitro by dysregulation of cell cycle progression due to altered proteasomal degradation of these cell cycle regulatory proteins, it could be as a candidate for the treatment of human cholangiocarcinoma either by itself or in combination with other chemotherapeutic agents.[1]
Tannic acid and rutin have lipid-lowering and antioxidative activities, can promote the excretion of fecal sterols, thereby leading to a decreased absorption of dietary cholesterol as well as lower plasma and hepatic cholesterol.[2]
TA treatment can inhibit the effect of collagenase digestion on dentin matrix, particularly for 10%TA and 20%TA, the TA-dentin matrix complex resulted in improved bond strength for both adhesive systems.[3]
Tannic acid has protective effects gainst hydrogen peroxide-induced oxidative stress and DNA damages in IMR-90 cells, suggests it can protect cells from oxidative stress.[4]
Tannic acid is a novel selective CXCL12/CXCR4 antagonist and consequently may provide a mechanistic basis for the reported antitumor and anti-inflammatory properties of tannic acid.[5]
Tannic acid has inhibition of multiplication of influenza virus.[6]
Product
References
[1] Marienfeld C, Tadlock L, Yamagiwa Y. Hepatology, 2003, 37(5):1097–104.
[2] Park S Y, Bok S H, Jeon S M, et al. Nutr Res, 2002, 22(3):283-95.
[3] Bedranrusso A K, Yoo K J, Ema K C, et al. J Dental Res, 2009, 88(9):807-11.
[4] Chen C H, Liu T Z, Chen C H, et al. Mol Nutr Food Res, 2007, 51(8):962–8.
[5] Chen X, Beutler JA, McCloud TG, et al. Clin Cancer Res , 2003, 9(8):3115-23.
[6] Green R H. J Clin Invest, 1948, 27(4):483-4.
[7] Wang Y, Qu F, Du Y, et al. Drug Standards of China, 2014,15(3):196-9.
Product Use Citation





