Natural Products
trans-Caryophyllene
| Catalog No. | CFN90502 | 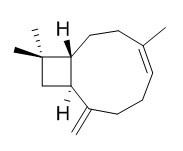 |
| CAS No. | 87-44-5 | |
| Molecular Weight: | 204.36 | |
| Molecular Formula | C15H24 | |
| DBs | [PubChem]:274954719 [ChEMBL]:5993 [PCIDB]:3110 |
Standard InChI:
InChI=1S/C15H24/c1-11-6-5-7-12(2)13-10-15(3,4)14(13)9-8-11/h6,13-14H,2,5,7-10H2,1,3-4H3/b11-6+/t13-,14-/m1/s1
Biological Activity
Trans-caryophyllene (TC), a component of essential oil found in many flowering plants, has shown its neuroprotective effects in various neurological disorders, TC has effect on kainic acid-induced seizure activity caused by oxidative stress and pro-inflammation, and significantly inhibits KA-induced generation of malondialdehyde, TC exert cerebral anti-inflammatory effects by mitigating the expression of proinflammatory cytokines, such as TNF-α and IL-1β; suggest that TC has a potential protective effect on chemical induced seizure and brain damage.[1]
Trans-caryophyllene and alpha-humulene from Salvia officinalis has cytotoxic activity in animal and human tumor cells.[2]
Trans-caryophyllene has anti-spasmodic activity on rat tracheal smooth muscle which could be explained, at least in part, by the voltage-dependent Ca²⁺ channels blockade.[3]
Trans-caryophyllene can reduce both acute and chronic pain in mice, which may be mediated through the opioid and endocannabinoid systems.[4]
Trans-caryophyllene possesses anti-inflammatory and analgesic properties, has the prevention of leukopenia in an experimental chemotherapy model in Wistar rats, exerts anti-inflammatory effects in TNF-α-stimulated chondrocyte models.[5,6]
Product
Official website: trans-Caryophyllene
Japanese website: trans-Caryophyllene
Chinese website: trans-Caryophyllene
Japanese website: trans-Caryophyllene
Chinese website: trans-Caryophyllene
References
[1] Liu H, Song Z, Liao D, et al. Neurochem Res, 2015, 40(1):118-23.
[2] Hadri A E, Rio M A G D, Sanz J, et al. An Real Acad F, 2010, 76(3):343-56.
[3] Pinho-Da-Silva L, Mendes-Maia P V, Teófilo T M, et al. Molecules, 2012, 17(10):11965-77.
[4] Paula-Freire L I G, Andersen M L, Gama V S, et al. Phytomed Int J Phytother Phytopharmacol, 2013, 21(3):356-62.
[5] Campos M I, Campos C N, Aarestrup F M. Mol Clin Oncol, 2015, 3(4):825-8.
[6] Campos M I C, Vieira W D A, Aarestrup F M, et al. Int J Mol Med, 2014, 34:S31.
[7] Patra K C, Singh B, Pareta S, et al. NatProd Res, 2010, 24(20):1933-8.
Product Use Citation





