Natural Products
Eurycomanone
| Catalog No. | CFN92008 | 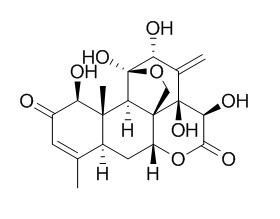 |
| CAS No. | 84633-29-4 | |
| Molecular Weight: | 408.4 | |
| Molecular Formula | C20H24O9 | |
| DBs | [PubChem]:274954384 [ChEMBL]:65887 [PCIDB]: |
Standard InChI:
InChI=1S/C20H24O9/c1-7-4-10(21)13(23)17(3)9(7)5-11-18-6-28-20(27,16(17)18)12(22)8(2)19(18,26)14(24)15(25)29-11/h4,9,11-14,16,22-24,26-27H,2,5-6H2,1,3H3/t9-,11+,12+,13+,14-,16+,17+,18+,19-,20?/m0/s1
Biological Activity
Eurycomanone has cytotoxic on HepG2 cells and Human Cervical Carcinoma Cells by inducing apoptosis through the up-regulation of p53 and Bax, and down-regulation of Bcl-2.[1,2]
Eurycomanone exerts antiproliferative activity via apoptosis in Hela cells MCF-7cells .[3,4] and
Eurycomanone at viable therapeutic concentrations of 5–20μg/ml exhibits significant anti-proliferative and anti-clonogenic cell growth effects on A549 lung cancer cells, the treatment also resulted in suppression of the lung cancer cell tumor markers and several known cancer cell growth-associated genes.[5]
Eurycomanone enhances testosterone steroidogenesis at the Leydig cells by inhibiting aromatase conversion of testosterone to oestrogen, and at a high concentration may also involve phosphodiesterase inhibition, it may be worthy for further development as a phytomedicine to treat testosterone-deficient idiopathic male infertility and sterility.[6]
Eurycomanone and eurycomanol as regulators of signaling pathways involved in proliferation, cell death and inflammation.[7]
Eurycomanone possesses growth inhibition of P. berghei by combination of eurycomanone-artesunate with doses 30 mg/kgBW-artesunate 4mg/kgBW, suggests that this combination can be used as potential antimalarial drug.[8]
Product
References
[1] Zakaria Y, Rahmat A, Pihie A H L, et al. Cancer Cell Int, 2009, 9(1):1-21.
[2] Mahfudh N, Pihie A H L. J Cancer Mol, 2008, 4(4):109-15.
[3] Nurkhasanah, Azimahtol Hawariah Lope Pihie,Jalifah Latip. Majalah Farmasi Indonesia, 2009, 20(4): 190-7.
[4] Pihie A H L. Anticancer Res, 2004, 24(5D):3426-7.
[5] Wong P F, Cheong W F, Shu M H, et al.Phytomedicine, 2012, 19(2):138-44.
[6] Low B S, Choi S B, Wahab H A, et al. J Ethnopharmacol, 2013, 149(1):201-7.
[7] Hajjouli S, Chateauvieux S, Teiten M H, et al. Molecules, 2014, 19(9):14649-66.
[8] Yusuf H, Satria D. J Chem Pharm Res, 2016,(82):18-22.
[9] Khari N, Aisha A, Ismail Z. Trop J Pharm Res, 2014, 13(5):801-7.
Product Use Citation





