Natural Products
Umbelliferone
| Catalog No. | CFN97503 | 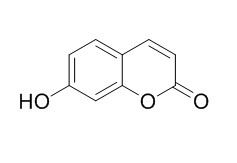 |
| CAS No. | 93-35-6 | |
| Molecular Weight: | 162.1 | |
| Molecular Formula | C9H6O3 | |
| DBs | [PubChem]:274952852 [ChEMBL]:27510 [PCIDB]: |
Standard InChI:
InChI=1S/C9H6O3/c10-7-3-1-6-2-4-9(11)12-8(6)5-7/h1-5,10H
Biological Activity
Umbelliferone (UMB), a natural antioxidant, is benzopyrone in nature, and it is present in the fruits of golden apple and bitter orange, UMB has a protective effect on membrane fatty acid composition of liver and kidney as supported by antioxidant and antihyperlipidemic effects of UMB reported earlier as evidenced by improved histopathological changes, hepatic and nephritic markers, indicating recovery from the risk of diabetic complications; UMB has antihyperglycemic effect, UMB at 30 mg/kg of body weight possesses a promising antihyperglycemic effect that is comparable with glibenclamide.[1,2]
Umbelliferone analogues and their potential to be antimutagenic/anticarcinogenic against mutations induced by benzo(a)pyrene, a potent mutagen/carcinogen, and hydrogen peroxide, and for their ability to function as free radical scavengers. [3]
Umbelliferone has antioxidative effect, the antioxidative effect is dose-dependent up to 100 ug/ml and then levelled off with no further increase in activity. [4]
Umbelliferone, ferulic acid and eugenol are competitive and limonene is a competitive–non-competitive inhibitor of alkaline phosphatase, ferulic acid and umbelliferone are competitive, whereas eugenol and limonene are competitive–non-competitive and uncompetitive inhibitors of acetylcholinesterase, respectively. [5]
Umbelliferone derivatives is a novel class of inhibitors for steroid 5α-reductase.[6]
Umbelliferone attenuates the alteration characteristics of allergic airway inflammation,
the mechanisms of action of this molecule may contribute for the development of new drugs for the treatment of asthma.[7]
Umbelliferone is an intracellular pH-sensitive fluorescent indicator and blood-brain barrier probe.[8]
Umbelliferone derivatives have antimicrobial properties, and has anti-mutagenic effects induced by 4-nitroquinoline 1-oxide or UV irradiation in E. Coli.[9,10]
Product
References
[1] Ramesh B, Viswanathan P, Pugalendi K V. Eur J Pharmacol, 2007, 566(1-3):231–9.
[2] Ramesh B, Pugalendi K V. J Med Food, 2006, 9(4):562-6.
[3] Pillai S P, Menon S R, Mitscher L A, et al. J Nat Prod, 1999, 62(10):1358-62.
[4] Rajbir Singh, Bikram Singh, Sukhpreet Singh,et al. Food Chem, 2010, 120(3):825-30.
[5] Kumar P, Singh V K, Singh D K. Phytother Res, 2009, 23(2):172–7.
[6] Fan G J, Mar W, Man K P, et al. Bioorg Med Chem Lett, 2001, 11(17):2361-3.
[7] Vasconcelos J F, Teixeira M M, Barbosa-Filho J M, et al. Eur J Pharm, 2009, 609:126-31.
[8] Jr S T, Anderson R E. J Neurophysiol, 1980, 44(1):60-75.
[9] Jurd L, King A D, Mihara K. Phytochemistry, 1971, 10(12):2965-70.
[10] Ohta T, Watanabe K, Moriya M, et al. Mutation Research/fundamental & Molecular Mechanisms of Mutagenesis, 1983, 117(1-2):135-8.
[11] Liu L X, Zhao T, Sun L X. Journal of Shenyang Pharmaceutical University, 2010, 27(7):563-6.
Product Use Citation





