Natural Products
Benzoylmesaconine
| Catalog No. | CFN98575 | 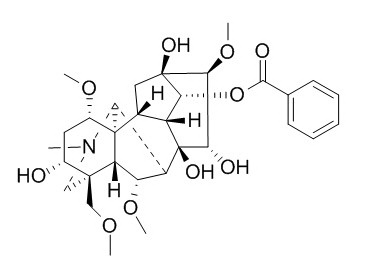 |
| CAS No. | 63238-67-5 | |
| Molecular Weight: | 589.68 | |
| Molecular Formula | C31H43NO10 | |
| DBs | [PubChem]:274951927 [ChEMBL]:132630 [PCIDB]: |
Standard InChI:
InChI=1S/C31H43NO10/c1-32-13-28(14-38-2)17(33)11-18(39-3)30-16-12-29(36)25(42-27(35)15-9-7-6-8-10-15)19(16)31(37,24(34)26(29)41-5)20(23(30)32)21(40-4)22(28)30/h6-10,16-26,33-34,36-37H,11-14H2,1-5H3/t16-,17-,18+,19-,20?,21+,22-,23-,24+,25-,26+,28+,29-,30+,31-/m1/s1
Biological Activity
Benzoylmesaconine (BEN, an aconitine derivative extracted from heated-Aconiti tuber) induces the generation of CD4+ T cells antagonistic to type 2 T cells (BEN-CD4+ T cells), the combination therapy of IL-12 (an inducer of type 1 T cell responses) and BEN (an inhibitor of type 2 T cell responses) may protect TI-mice from severe HSV-1 infection.
Benzoylmesaconine has antinociceptive effects.[1]
Benzoylmesaconine has inhibition effects on opportunistic herpesvirus infections in mice with acquired immunodeficiency syndrome.[2]
Product
Official website: Benzoylmesaconine
Japanese website: Benzoylmesaconine
Chinese website: Benzoylmesaconine
Japanese website: Benzoylmesaconine
Chinese website: Benzoylmesaconine
References
[1] Kobayashi M, Takahashi H, Herndon D N, et al. Burns, 2003, 29(1):37-42.
[2] Suzuki Y, Oyama T, Ishige A, et al. Planta Med, 1994, 60(5):391-4.
[3] Ball Ma, Kobayashi, Polland R B, et al. J Immunother, 1995, 17(2):127.
[4] Nie J, Zhang L, Tian S. China Pharmacist, 2003, 6(01):38-9.
Product Use Citation





