Natural Products
D-Pinitol
| Catalog No. | CFN99044 | 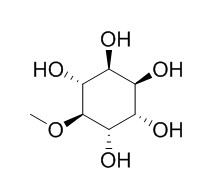 |
| CAS No. | 10284-63-6 | |
| Molecular Weight: | 194.2 | |
| Molecular Formula | C7H14O6 | |
| DBs | [PubChem]:274951463 [ChEMBL]:28548 [PCIDB]: |
Standard InChI:
InChI=1S/C7H14O6/c1-13-7-5(11)3(9)2(8)4(10)6(7)12/h2-12H,1H3/t2?,3-,4-,5-,6+,7?/m0/s1
Biological Activity
D-Pinitol is azole nucleoside analogue, as potential antitumor agents; it reduces the migration and the invasion of prostate cancer cells (PC3 and DU145) at noncytotoxic concentrations, reduces mRNA and cell surface expression of αvβ3 integrin, exerts its inhibitory effects by reducing focal adhesion kinase (FAK) phosphorylation, c-Src kinase activity and NF-kB activation; thus, D-pinitol may be a novel anti-metastasis agent for the treatment of prostate cancer metastasis.[1,2]
D-Pinitol has hepatoprotective effects by attenuating hyperglycaemia-mediated pro-inflammatory cytokines and oxidative stress.[3]
D-Pinitol and myo-inositol stimulate translocation of glucose transporter 4 in skeletal muscle of C57BL/6 mice, they have the potential to prevent diabetes mellitus by reducing the postprandial blood glucose level and stimulating GLUT4 translocation in the skeletal muscle.[4]
D-Pinitol significantly inhibits the proliferation of MCF-7 cells in a concentration-dependent manner, while upregulating the expression of p53, Bax and down regulating Bcl-2 and NF-kB, thus, D-pinitol induces apotosis in MCF-7 cells through regulation of proteins of pro- and anti-apoptotic cascades.[5]
D-Pinitol efficiently attenuates the hazardous consequences of the environmental carcinogen 7,12-DMBA through modulating cell surface glycoproteins, membrane protective role both in lysosomal and ATPase compartment via its antioxidant nature which ultimately results in the findings of future innovative remedies for genotoxin mediated hazards.[6]
Product
References
[1] Tianrong Z, Hongxiang L. Carbohyd Res, 2007, 342(6):865-9.
[2] Lin T H, Tan T W, Tsai T H, et al. Int J Mol Sci, 2013, 14(5):9790-802.
[3] Sivakumar S, Palsamy P, Subramanian S P. Free Radical Res, 2010, 44(6):668-78.
[4] Dang N T, Mukai R, Yoshida K, et al. Biosci Biotech Biochem, 2010, 74(5):1062-7.
[5] Rengarajan T, Nandakumar N, Rajendran P, et al. Asian Pac J Cancer P, 2014, 15(4):1757-62.
[6] Rengarajan T, Nandakumar N, Balasubramanian M P. J Exp Ther Oncol, 2012, 10(1):39-49.
[7] Ling C, Jian H, Yang L V, et al. Chinese Journal of Experimental Traditional Medical Formulae, 2011, 17(5):80-2.
Product Use Citation





