Natural Products
Glycitein
| Catalog No. | CFN99106 | 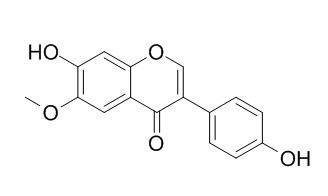 |
| CAS No. | 40957-83-3 | |
| Molecular Weight: | 284.26 | |
| Molecular Formula | C16H12O5 | |
| DBs | [PubChem]:274951312 [ChEMBL]:34778 [PCIDB]: |
Standard InChI:
InChI=1S/C16H12O5/c1-20-15-6-11-14(7-13(15)18)21-8-12(16(11)19)9-2-4-10(17)5-3-9/h2-8,17-18H,1H3
Biological Activity
Glycitein accounts for 5-10% of the total isoflavones in soy food products, has weak estrogenic activity, comparable to that of the other soy isoflavones but much lower than that of DES and 17beta-estradiol.[1]
Glycitein, daidzein and glenistein, with their inhibitory effects on natural and PDGF-BB-induced SMC proliferation, may be useful in attenuating such proliferation, a basic mechanism involved in atherosclerotic vascular change, thereby preventing atherosclerotic cardiovascular diseases.[2]
Glycitein has antioxidant effects, may suppress Abeta toxicity through combined antioxidative activity and inhibition of Abeta deposition, thus may have therapeutic potential for prevention of Abeta associated neurodegenerative disorders.[3]
Glycitein has inhibitory effects on hydrogen peroxide induced cell damage by scavenging reactive oxygen species and inhibiting c-Jun N-terminal kinase.[4]
Glycitein, the most potent activator of ERK1/2, decreases RWPE-1 cell proliferation by 40% ; it induces ERK1/2 activation was dependent, in part, on tyrosine kinase activity associated with vascular endothelial growth factor receptor (VEGFR). [5]
Glycitein suppresses PMA-induced phosphorylation of three types of MAP kinases, which are upstream signaling molecules in MMP gene expressions and NF-kappaB and AP-1 activities in glioma cells, therefore, the inhibition of MMP-3 and MMP-9 expression by glycitein may have therapeutic potential for controlling invasiveness of malignant gliomas.[6]
Product
References
[1] T. Song T, Suzanne Hendrich A, Murphy P A. J Agr Food Chem, 1999, 47(4):1607-10.
[2] Pan W, Ikeda K, Takebe M, et al. J Nut, 2001, 131(4):1154-8.
[3] Gutierrez-Zepeda A, Santell R, Wu Z, et al. Bmc Neurosci, 2004, 6(3):1-9.
[4] Kang K A, Zhang R, Piao M J, et al. Free Radical Res, 2007, 41(6):720-9.
[5] Clubbs E A, Bomser J A. J Nut Biochem, 2007, 18(8):525-32.
[6] Lee E J, Kim S Y, Hyun J W, et al. Chem Biol Interact, 2010, 185(1):18-24.
[7] César I D C, Braga F C, Vianna-Soares C D, et al. Rev.Bras.Farmacogn, 2007, 17(4):616-25.
Product Use Citation





