Natural Products
Mesaconitine
| Catalog No. | CFN99199 | 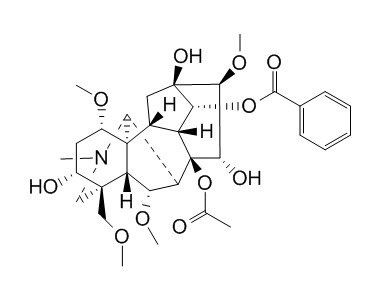 |
| CAS No. | 2752-64-9 | |
| Molecular Weight: | 631.71 | |
| Molecular Formula | C33H45NO11 | |
| DBs | [PubChem]:274950951 [ChEMBL]:6773 [PCIDB]: |
Standard InChI:
InChI=1S/C33H45NO11/c1-16(35)45-33-21-18(13-31(39,28(43-6)26(33)37)27(21)44-29(38)17-10-8-7-9-11-17)32-20(41-4)12-19(36)30(15-40-3)14-34(2)25(32)22(33)23(42-5)24(30)32/h7-11,18-28,36-37,39H,12-15H2,1-6H3/t18-,19-,20+,21-,22?,23+,24-,25-,26+,27-,28+,30+,31-,32+,33-/m1/s1
Biological Activity
Mesaconitine increases the [Ca2+]i level in endothelial cells by influx of Ca2+ from extracellular spaces, suggests that mesaconitine-induced Ca2+ influx and activation of nitric-oxide synthase in endothelial cells and, thus, induced vasorelaxation in rat aorta.[1]
Mesaconitine is highly toxic, can inhibit Efflux transporters, including P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and multidrug resistance-associated protein isoform 2 (MRP2).[2]
Mesaconitine has antinociceptive activity, has inhibition of stimulus-triggered and spontaneous epileptiform activity in rat hippocampal slices.[3,4]
Mesaconitine has antiinflammatory activity, can inhibit carrageenin-induced hind-paw edema in sham-operated mice as well as adrenalectomized mice, it do not affect the biosynthesis of the prostaglandins. [5]
Product
References
[1] Mitamura M, Horie S, Sakaguchi M, et al. Eur J Pharmacol, 2013, 436(3):217-25.
[2] Ling Y, Yang X, Zhen Y, et al. Toxicol Lett, 2013, 216(2-3):86-99.
[3] Suzuki Y, Oyama T, Ishige A, et al. Planta Med, 1994, 60(5):391-4.
[4] Ameri A. Eur J Pharmacol, 1998, 342(2-3):183-91.
[5] Hiroshi H, Hiroshi T, Mitsuo F, et al. Eur J Pharmacol, 1982, 82(1-2):65-71.
[6] Zhou J, Ling Y E, Tang L, et al. China Journal of Traditional Chinese Medicine & Pharmacy, 2013, 38(10):1521-5.
Product Use Citation





