Natural Products
Silymarin
| Catalog No. | CFN99542 | 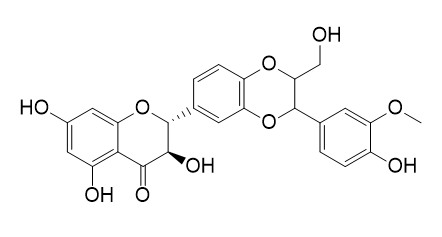 |
| CAS No. | 22888-70-6 | |
| Molecular Weight: | 482.46 | |
| Molecular Formula | C25H22O10 | |
| DBs | [PubChem]:274950611 [ChEMBL]: [PCIDB]: |
Standard InChI:
InChI=1S/C25H22O10/c1-32-17-6-11(2-4-14(17)28)24-20(10-26)33-16-5-3-12(7-18(16)34-24)25-23(31)22(30)21-15(29)8-13(27)9-19(21)35-25/h2-9,20,23-29,31H,10H2,1H3/t20-,23+,24-,25-/m1/s1
Biological Activity
Silymarin (SIL), a standardized plant extract containing about 60% polyphenole silibinin, is used as a hepatoprotective agent, it retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats, it also may play a role in the therapy of (alcoholic) liver cirrhosis.[1,2]
Silymarin modulates imbalance between cell survival and apoptosis through interference with the expressions of cell cycle regulators and proteins involved in apoptosis; it also shows anti-inflammatory as well as anti-metastatic activity; it has the protective effects in various tissues, suggest a clinical application in cancer patients as an adjunct to established therapies, to prevent or reduce chemotherapy as well as radiotherapy-induced toxicity.[3]
Silymarin possesses antioxidant, anti-inflammatory and immunomodulatory properties which may lead to the prevention of skin cancer in in vivo animal models, suggests that it is a promising chemopreventive and pharmacologically safe agent which can be exploited or tested against skin cancer in human system, moreover, it may favorably supplement sunscreen protection and provide additional anti-photocarcinogenic protection.[4]
Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation.[5]
Silymarin and silibinin cause G1 and G2–M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells.[6]
Product
References
[1] Boigk G, Stroedter L, Herbst H, et al. Hepatology, 1997, 26(3):643-9.
[2] Saller R, Meier R, Brignoli R. Drugs, 2001, 61(14):2035-63.
[3] Ramasamy K, Agarwal R. Cancer Lett, 2008, 269(269):352-62.
[4] Katiyar S K. Int J Oncol, 2005, 26(1):169-76.
[5] Katiyar S K, Roy A M, Baliga M S. Mol Cancer Ther, 2005, 4(2):207-16.
[6] Deep G, Singh R P, Agarwal C, et al. Oncogene, 2006, 25(7):1053-69.
[7] Korany M A, Haggag R S, Ragab M A A, et al. Arab J Chem, 2013, 06.021.
Product Use Citation





