Natural Products
Procyanidin B2
| Catalog No. | CFN99558 | 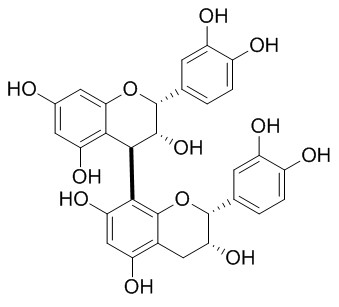 |
| CAS No. | 29106-49-8 | |
| Molecular Weight: | 578.52 | |
| Molecular Formula | C30H26O12 | |
| DBs | [PubChem]:274950595 [ChEMBL]:75632 [PCIDB]:9077 |
Standard InChI:
InChI=1S/C30H26O12/c31-13-7-20(37)24-23(8-13)41-29(12-2-4-16(33)19(36)6-12)27(40)26(24)25-21(38)10-17(34)14-9-22(39)28(42-30(14)25)11-1-3-15(32)18(35)5-11/h1-8,10,22,26-29,31-40H,9H2/t22-,26-,27-,28-,29-/m1/s1
Biological Activity
Procyanidin B2 (PB2) is a naturally occurring flavonoid widely found in cocoa, red wine and grape juice, PB2 could protect against oxidative stress- and chemical-induced injury in colonic cells by modulating the endogenous cellular defence, PB2 protects against oxidative injury in colonic cells and up-regulate the expression of GSTP1 via a mechanism that involves ERK and p38 MAPK activation and Nrf2 translocation.[1]
Procyanidin B2 (PB2) is one of phenolic compounds in apple pomace, an agro-industrial byproduct in apple juice processing, PB2 at no less than 50 mu g.mL(-1) could significantly suppress inflammation in the LPS-induced cells, shows that high pure PB2 prepared from apple pomace has a remarkable anti-inflammatory property.[2]
Procyanidin B2 exhibits cytotoxic activity to MCF-7 cells and it could be a potential antineoplastic agent. [3]
Procyanidin B2 has toxic property towards triple negative breast cancer cells, it may shows new promise for therapeutic intervention of cancer.[4]
Procyanidin B2( PB2) is absorbed and excreted in urine, and a portion of the PB2 is degraded to (−)-epicatechin and to the metabolized conjugated and/or methylated (−)-epicatechin internally in the rat, PB2 also can reduces the accumulation of lipid peroxide in plasma oxidized by copper ions.[5]
Procyanidin B2 and a cocoa polyphenolic extract inhibit acrylamide-induced apoptosis in human Caco-2 cells by preventing oxidative stress and activation of JNK pathway.[6]
Procyanidin B2 has anti- and pro-oxidant effects on metal-mediated DNA damage by interacting with H2O2 and metal ions.[7]
Product
References
[1] Rodríguez-Ramiro I, Ramos S, Bravo L, et al.Eur J Nutr, 2012, 51(7):881-92.
[2] Zhang H, Ying C. Bangl J Pharmacol, 2011, 6(2):106-10.
[3] Avelar M M, Gouvêa C M. Indian J Pharml Sci, 2012, 74(4):351-5.
[4]Shilpi A, Parbin S, Sengupta D, et al. Chem-biol Intact, 2015, 233:122-38.
[5] Seigo Baba, Naomi Osakabe, Midori Natsume, et al. Free Radical Bio Med, 2002, 33(1):142-8.
[6] Ramos S, Bravo L, Goya L, et al. J Nutr Biochem, 2011, 22(12):1186-94.
[7] Sakano K, Mizutani M, Murata M, et al. Free Radical Biol Med, 2005, 39(8):1041-9.
[8] Cheng X G, Wen-Zheng J U, Dai G L, et al. Pharm Clin Res, 2013, 21(01):39-41.
Product Use Citation





