Natural Products
Atractylenolide III
| Catalog No. | CFN99946 | 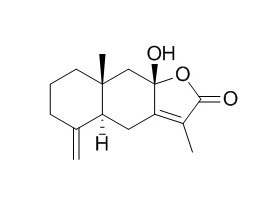 |
| CAS No. | 73030-71-4 | |
| Molecular Weight: | 248.32 | |
| Molecular Formula | C15H20O3 | |
| DBs | [PubChem]:274951008 [ChEMBL]:69958 [PCIDB]: |
Standard InChI:
InChI=1S/C15H20O3/c1-9-5-4-6-14(3)8-15(17)12(7-11(9)14)10(2)13(16)18-15/h11,17H,1,4-8H2,2-3H3/t11-,14+,15-/m0/s1
Biological Activity
Atractylenolide III is the major bioactive component of Atractylodes lancea, it inhibits histamine release, suppresses activation of p38 mitogen-activated protein kinase, C-Jun-N-terminal protein kinase, and nuclear factor-κB in stimulated HMC-1 cells, and suppresses the activation of caspase-1 and the expression of receptor interacting protein-2, suggests that atractylenolide III may control immunological reactions by regulating the cellular functions of IL-6 in mast cells.[1]
Atractylenolide III and atractylenolide I have anti-inflammatory activity through inhibition of nuclear factor-κB and mitogen-activated protein kinase pathways in mouse macrophages, can inhibit Lipopolysaccharide-induced TNF- α and NO production in macrophages.[2,3]
Atractylenolide III can induce apoptosis in human lung carcinoma A549 cells via mitochondria-mediated death pathway, indicates that it is a potential candidate for treatment of human lung carcinoma.[4]
Atractylenolide III (LD50, 103.3 mg/m2) and atractylon (136.2 mg/m2) are potential house dust mite control agents, they are five and four times more toxic than Deet and 1.7- and 1.3-fold more active than dibutyl phthalate, respectively, based on 24 h LD50 values. [5]
Atractylenolide III has neuroprotection against glutamate-induced neuronal apoptosis via inhibiting caspase signaling pathway.[6]
Atractylenolide III has gastroprotective activity on ethanol-induced gastric ulcer in vitro and in vivo.[7]
Product
Official website: Atractylenolide III
Japanese website: Atractylenolide III
Chinese website: Atractylenolide III
Japanese website: Atractylenolide III
Chinese website: Atractylenolide III
References
[1] Kang T H, Han N R, Kim H M, et al. J Nat Prod, 2011, 74(2):223-7.
[2]Li C Q, He L C, Jin J Q. Phytother Res, 2007, 21(4):347–53.
[3] Ji G Q, Chen R Q, Wang L. Immunopharmacol Immunot, 2015,38(2):98-102.
[4] Kang T H, Bang J Y, Kim M H, et al. Food Chem Toxicol , 2011, 49(2):514-9.
[5] Kim H K, Yun Y K, Ahn Y J. J Agri Food Chem, 2007, 55(15):6027-31.
[6] Liu C, Zhao H, Ji Z H, et al. Neurocheml Res, 2014, 39(9):1753-8.
[7] Wang K T, Chen L G, Wu C H, et al. J Pharm Pharmacol, 2010, 62(3):381-8.
[8] Ying Guo, Yong Chun Jin,Ke Yuan,et al. Asian Journal of Chemistry,2012, 24(10):4425-8.
Product Use Citation





