Natural Products
Formononetin
| Catalog No. | CFN99962 | 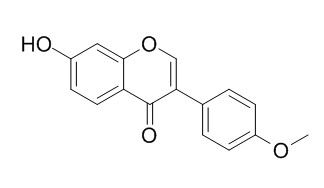 |
| CAS No. | 485-72-3 | |
| Molecular Weight: | 268.27 | |
| Molecular Formula | C16H12O4 | |
| DBs | [PubChem]:4115 [ChEMBL]:18088 [PCIDB]:2525 |
Standard InChI:
InChI=1S/C16H12O4/c1-19-12-5-2-10(3-6-12)14-9-20-15-8-11(17)4-7-13(15)16(14)18/h2-9,17H,1H3
Biological Activity
Formononetin causes vascular relaxation via endothelium/NO-dependent mechanism and endothelium-independent mechanism which involves the activation of BK(Ca) and K(ATP) channels.[1]
Formononetin-treated Ovx rats has an increased bone osteoprotegerin-to-receptor activator for nuclear κB ligand ratio compared with the Ovx + vehicle group; daily oral administration of formononetin for 12 weeks has a substantial anabolic effect, thus raising the possibility of its use in postmenopausal osteoporosis.[2]
Formononetin exhibits antiviral activities against some members of Picornaviridae, could inhibit EV71-induced COX-2 expression and PGE2 production via MAPKs pathway including ERK, p38 and JNK, thus, formononetin could be a potential lead or supplement for the development of new anti-EV71 agents in the future.[3]
Formononetin reduces hydrogen peroxide (H2O2)-induced apoptosis and improves the levels or activity of indicators of oxidative stress, also inhibits the activation of nuclear factor-kappaB (NF-κB), which is a significant transcription factor for RGC-5 apoptosis.[4]
Product
References
[1] Wu J H, Li Q, Wu M Y, et al. J Nutr Biochem, 2010, 21(7):613-20.
[2]Tyagi A M, Srivastava K, Singh A K, et al. Menopause, 2012, 19(8):856-63.
[3] Wang H, Zhang D, Miao G, et al. Viro J, 2015, 12(1):1-10.
[4] Jia W C, Liu G, Zhang C D, et al. Eur Rev Med & Pharmaco, 2014, 18(15):2191-7.
[5] Xing J H, Sun X L, Zhou J.Chinese Journal of Pharmaceutical Analysis, 2009(01):73-5.
Product Use Citation





