Natural Products
Ginsenoside Rg2
| Catalog No. | CFN99968 | 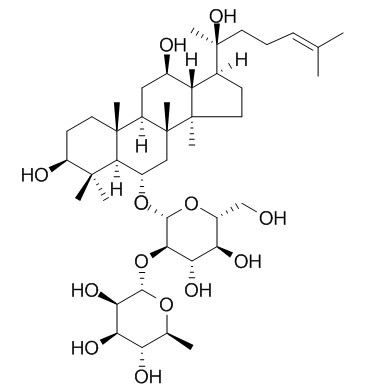 |
| CAS No. | 52286-74-5 | |
| Molecular Weight: | 785.02 | |
| Molecular Formula | C42H72O13 | |
| DBs | [PubChem]:274950986 [ChEMBL]:77151 [PCIDB]:30399 |
Standard InChI:
InChI=1S/C42H72O13/c1-20(2)11-10-14-42(9,51)22-12-16-40(7)28(22)23(44)17-26-39(6)15-13-27(45)38(4,5)35(39)24(18-41(26,40)8)53-37-34(32(49)30(47)25(19-43)54-37)55-36-33(50)31(48)29(46)21(3)52-36/h11,21-37,43-51H,10,12-19H2,1-9H3/t21-,22-,23+,24-,25+,26+,27-,28-,29-,30+,31+,32-,33+,34+,35-,36-,37+,39+,40+,41+,42-/m0/s1
Biological Activity
Ginsenoside Rg2 suppresses the hepatic glucose production via AMPK-induced phosphorylation of GSK3β and induction of SHP gene expression, suggests that it has therapeutic potential for type 2 diabetic patients.[1]
Ginsenoside Rg2 inhibits nicotinic acetylcholine receptor-mediated Na+ influx and channel activity; it also inhibits the 5-HT-induced inward peak current (I5-HT) in dose dependent and reversible manner, the half-inhibitory concentrations (IC50) of ginsenoside Rg2 is 22.3 +/- 4.6 microM, suggests that it might regulate the 5-HT3A receptors that are expressed in Xenopus oocytes.[2]
Ginsenoside Rg2 can reduce LPS-mediated THP-1 monocyte adhesion to HUVEC, in a concentration-dependent manne, it may provide direct vascular benefits with inhibition of leukocyte adhesion into vascular wall thereby providing protection against vascular inflammatory disease.[3]
Ginsenoside Rg2 protects cells against UVB-induced genotoxicity by increasing DNA repair, in possible association with modulation of protein levels involved in p53 signaling pathway.[4]
Ginsenoside Rg2 improves learning and memory through mechanisms related to anti-apoptosis in MID rats, indicates that it may represent a potential neurorestorative treatment strategy for vascular dementia or other ischemic insults.[5]
Ginsenoside Rg2 has protective effects against H2O2-induced injury and apoptosis in H9c2 cells.[6]
Product
Official website: Ginsenoside Rg2
Japanese website: Ginsenoside Rg2
Chinese website: Ginsenoside Rg2
Japanese website: Ginsenoside Rg2
Chinese website: Ginsenoside Rg2
References
[1] Yuan H D, Kim D Y, Quan H Y, et al. Chem-Bioll Interact, 2012, 195(1):35-42.
[2] Choi S, Lee J H, Oh S, et al. Mol Cells, 2003, 15(1):108-13.
[3] Cho Y S, Chan H K, Ha T S, et al. Korean J Physiol Pham 2013, 17(2):133-7.
[4] Ha S E, Shin D H, Kim H D, et al. N-S Arch Pharmacol, 2010, 382(1):89-101.
[5] Gong Z H, Liu M X, Gong L L, et al. Prog in Modern Biomed, 2010,10(06):1069-75.
[6] Fu W, Sui D, Yu X, et al. Int J Clin Exp Med, 2015, 8(11):19938-47.
[7] Yu M, Mi H, Jiao L. China Pharmacist, 2005, 8(12):1017-9.
Product Use Citation





