Natural Products
Ginsenoside Rg3
| Catalog No. | CFN99969 | 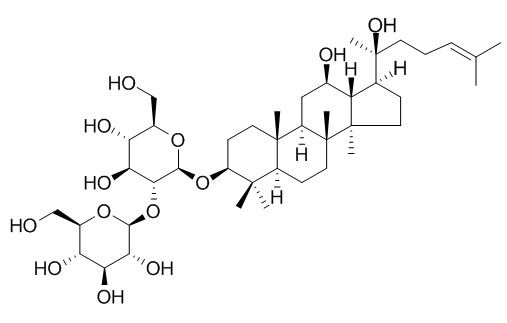 |
| CAS No. | 14197-60-5 | |
| Molecular Weight: | 785.02 | |
| Molecular Formula | C42H72O13 | |
| DBs | [PubChem]:254741245 [ChEMBL]:67991 [PCIDB]:30400 |
Standard InChI:
InChI=1S/C42H72O13/c1-21(2)10-9-14-42(8,51)22-11-16-41(7)29(22)23(45)18-27-39(5)15-13-28(38(3,4)26(39)12-17-40(27,41)6)54-37-35(33(49)31(47)25(20-44)53-37)55-36-34(50)32(48)30(46)24(19-43)52-36/h10,22-37,43-51H,9,11-20H2,1-8H3/t22-,23+,24+,25+,26-,27+,28-,29-,30+,31+,32-,33-,34+,35+,36-,37-,39-,40+,41+,42-/m0/s1
Biological Activity
Ginsenoside-Rg3, possess an ability to inhibit the lung metastasis of tumor cells, and the mechanism of their antimetastatic effect is related to inhibition of the adhesion and invasion of tumor cells, and also to anti-angiogenesis activity.[1]
Ginsenoside-Rg3 (2.5 or 5.0 mg/kg body weight,s.c. injections) inhibits cancer metastasis through activities that do not affect the growth or vascularity of intestinal cancers.[2]
Ginsenoside Rg3 has anti-tumor property through its angiosuppressive activity, is a ginsenoside monomer with high anti-cancer activity.[3,4]
Ginsenoside-Rg3 has new pharmacological activity against testosterone-induced prostate overgrowth,downregulates AR by facilitating the degradation of AR protein, could be potential therapeutic regimens for treating BPH.[5]
Ginsenoside-Rg3 is a novel drug, capable of inhibiting the early of scarring (HS) and later HS. GS-Rg3/electrospun is a very promising new treatment for early and long-term treatment of HS.[6]
Product
Official website: Ginsenoside Rg3
Japanese website: Ginsenoside Rg3
Chinese website: Ginsenoside Rg3
Japanese website: Ginsenoside Rg3
Chinese website: Ginsenoside Rg3
References
[1] Mochizuki M, Yoo Y C, Matsuzawa K, et al. Biol Pharm Bull, 1995, 18(9):1197-202.
[2] Iishi H, Tatsuta M, Baba M, et al. Clin Exp Metastas , 1997, 15(6):603-11.
[3] Yue P Y K, Wong D Y L, Wu P K, et al. Biochem Pharmcol, 2006, 72(4):437-45.
[4] Xue-Long L I, Yao-Yao F U, Sun S Y, et al. Journal of Dalian Polytechnic University, 2010,29(6):396-8.
[5] Bae J S, Park H S, Park J W, et al. J Nat Med, 2012, 66(3):476-85.
[6] Cheng L, Sun X, Hu C, et al. Acta Biomater, 2013, 9(12):9461-73.
[7] Zhao Q, Zheng X, Jiang J, et al. J Chromatogr B , 2010, 878(24): 2266-73.
Product Use Citation





