Natural Products
Ginsenoside Rf
| Catalog No. | CFN99976 | 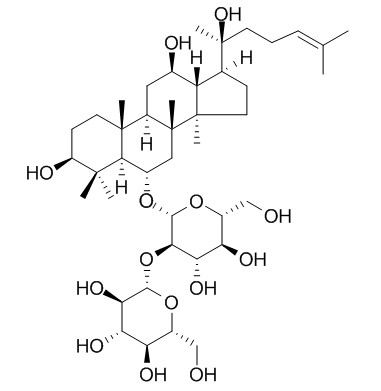 |
| CAS No. | 52286-58-5 | |
| Molecular Weight: | 801.01 | |
| Molecular Formula | C42H72O14 | |
| DBs | [PubChem]:274950978 [ChEMBL]:67986 [PCIDB]:3519 |
Standard InChI:
InChI=1S/C42H72O14/c1-20(2)10-9-13-42(8,52)21-11-15-40(6)28(21)22(45)16-26-39(5)14-12-27(46)38(3,4)35(39)23(17-41(26,40)7)53-37-34(32(50)30(48)25(19-44)55-37)56-36-33(51)31(49)29(47)24(18-43)54-36/h10,21-37,43-52H,9,11-19H2,1-8H3/t21-,22+,23-,24?,25?,26+,27-,28-,29+,30+,31-,32?,33?,34?,35-,36-,37+,39+,40+,41+,42-/m0/s1
Biological Activity
GinsenosideRf, as an effective natural product, induces G2/Mphase cell cycle arrest and apoptosis in human osteosarcoma MG-63 cells through the mitochondrial pathway, suggests that it may have a therapeutic effect on human osteosarcoma.[1]
Ginsenoside Rf(Rf) , a trace component of ginseng root, produces antinociception in mice; Ginsenoside Rf potentiates U-50,488H-induced analgesia and inhibits tolerance to its analgesia in mice.[2,3]
Ginsenoside Rf regulates voltage-dependent Ca(2+) channels through pertussis toxin (PTX)-sensitive G proteins in rat sensory neurons, suggests that Rf can act through a novel G protein-linked receptor in the nervous system. [4]
Ginsenoside Rf induces CYP3A4 and MDR1 gene expression through constitutive androstane receptor- and pregnane X receptor-mediated pathways. [5]
Ginsenoside Rf significantly reduces the production of IL-1β, IL-6, TNF-α, NO, and ROS, which are most highly activated in inflammatory bowel disease (IBD), and suppresses TNF-α/LPS-induced NF-κB transcriptional activity; suggests that ginsenoside Rf has potent intestinal anti-inflammatory effects that could be used to treat diseases such as IBD.[6]
Product
References
[1] Shangguan W J, Li H, Zhang Y H. Oncol Rep, 2014, 31(1):305-13.
[2] Mogil J S, Shin Y H, Mccleskey E W, et al. Brain Res, 1998, 792(2):218-28.
[3] Nemmani K V S, Ramarao P. Life Sci, 2003, 72(7):759-68.
[4] Choi S, Jung S Y, Ko Y S, et al. Mol Pharmacol, 2002, 61(4):928-35.
[5] Li Y, Wang Q, Yao X, et al. Eur J Pharmacol, 2010, 640(1-3):46-54.
[6] Ahn S, Siddiqi M H, Aceituno V C, et al. Immunl Invest, 2016,45(5):439-49.
[7] Zang P, Zhang P, Gao Y, et al. J Med Plant Res, 2011, 5(23):5513-6.
Product Use Citation





