| Kinase Assay: |
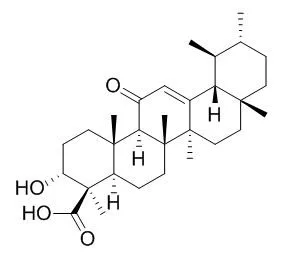
| Mol Carcinog. 2012 Sep;51(9):679-95. | | A novel cyano derivative of 11-keto-β-boswellic acid causes apoptotic death by disrupting PI3K/AKT/Hsp-90 cascade, mitochondrial integrity, and other cell survival signaling events in HL-60 cells.[Pubmed: 21751262] | Intervention of apoptosis is a promising strategy for discovery of novel anti-cancer therapeutics.
In this study, we examined the ability of a novel cyano derivative of 11-Keto-beta-boswellic acid , that is, butyl 2-cyano-3,11-dioxours-1,12-dien-24-oate (BCDD) to induce apoptosis in cancer cells.
METHODS AND RESULTS:
BCDD inhibited cell proliferation with 48 h IC(50) of 0.67 μM in HL-60, 1 μM in Molt4, and 1.5 μM in THP1 cells. The mechanism of cell death was investigated in HL-60 cells where it caused apoptosis by acting against several potential apoptosis suppressive targets. It inhibited phosphatidylinositol-3-kinase (PI3K)/AKT activity, NF-κB, Hsp-90, and survivin which may enhance the sensitivity of cells to apoptosis. Also, BCDD decreased the activity of Bid and Bax in cytosol, caused ΔΨ(mt) loss, releasing pro-apoptotic cytochrome c, SMAC/DIABLO leading to caspase-9-mediated down stream activation of caspase-3, ICAD, and PARP1 cleavage. Translocation of apoptotis-inducing factor (AIF) from mitochondria to the nucleus indicated some caspases-independent apoptosis. Though it upregulated DR-5 and caspase-8, the caspase inhibitor yet had no effect on apoptosis as against 75% inhibition by caspase-9 inhibitor. Attempts were made to examine any acclaimed role of AIF in the activation of caspase-8 using siRNA where it had no effect on caspase-8 activity while the Bax-siRNA inhibited caspase-3 activation suggesting predominance of intrinsic signaling.
CONCLUSIONS:
Our studies thus demonstrated that BCDD exerts multi-focal action in cancer cells while it required 10-fold higher the concentration to produce cytotoxicity in normal human PBMC and gingival cell line, and therefore, may find usefulness in the management of human leukemia. |
|
| Cell Research: |
| Chem Biol Interact. 2011 Jan 15;189(1-2):60-71. | | A propionyloxy derivative of 11-keto-β-boswellic acid induces apoptosis in HL-60 cells mediated through topoisomerase I & II inhibition.[Pubmed: 21056033 ] | Boswellic acids have invariably been reported for their antiproliferative potential in various cell systems. In the present study the growth inhibitory effect of propionyloxy derivative of 11-Keto-beta-boswellic acid (PKBA; a semisynthetic analogue of 11-keto-β-boswellic acid) on HL-60 promyelocytic leukemia cells is being reported for the first time.
METHODS AND RESULTS:
In the preliminary studies, in vitro cytotoxicity of PKBA was investigated against eight human cancer cell lines viz., IMR-32, SF-295 (both neuroblastoma), PC-3 (prostate), Colo-205 (colon), MCF-7 (breast), OVCAR-5 (ovary), HL-60, Molt-4 (both leukemia) and their respective IC(50) values were found to be 5.95, 7.11, 15.2, 14.5, 15, 15.9, 8.7 & 9.5μg/ml, respectively. For determining the mechanism of cell death in HL-60 cells, PKBA was subjected to different mechanistic studies. DNA relaxation assay of PKBA revealed inhibition of both topoisomerases I & II. The fragmentation analysis of DNA revealed typical ladders indicating the cytotoxic effect to be mediated by induction of apoptosis. The morphologic studies of PKBA showed the presence of true apoptotic bodies. Apoptosis was confirmed further by flow-cytometric detection of sub-G(1) peaks and enhanced annexin-V-FITC binding of the cells. The activation of apoptotic cascade by PKBA in HL-60 cells was found to be associated with the loss of mitochondrial membrane potential, release of cytochrome c, activation of initiator and executioner caspases and cleavage of poly ADP ribose polymerase (PARP). In vivo studies of PKBA revealed anti-tumoral activity against both ascitic and solid murine tumor models.
CONCLUSIONS:
These studies thus demonstrate PKBA to induce apoptosis in HL-60 cells due to the inhibition of topoisomerases I and II. |
|
| Animal Research: |
| Naunyn Schmiedebergs Arch Pharmacol. 2013 Sep;386(9):823-33. | | The selective 5-LOX inhibitor 11-keto-β-boswellic acid protects against myocardial ischemia reperfusion injury in rats: involvement of redox and inflammatory cascades.[Pubmed: 23771412] | Myocardial ischemia induces 5-lipoxygenase (LOX) translocation and leukotriene production in the heart. Leukotrienes increase inflammatory responses aggravating, thereby, ischemia-reperfusion (I/R) injury. This study aimed to investigate whether the selective 5-LOX inhibitor 11-Keto-beta-boswellic acid (11-keto BA), in three different dose levels, exert a protective effect on myocardial I/R injury in an in vivo rat heart model.
METHODS AND RESULTS:
Sixty male Wister rats were used in this study and divided into five equal groups (n=12): GP1, sham-operated receiving normal saline; Gp 2, rats were subjected to 45 min left anterior descending coronary artery ligation followed by 4 h reperfusion to serve as I/R group. Gps 3-5 received 11-keto BA in doses 250, 500, 1,000 mg/kg, respectively, via an oral gavage for 7 days then were exposed to I/R. I/R injury induced a significant elevation in myeloperoxidase activity and gene expression of intracellular adhesion molecules, cyclooxygenase-2, 5-lipooxygenasae, nuclear factor kappa-beta, tumor necrosis factor alpha, nuclear factor (erythroid-derived 2)-like 2, and hemeoxygenease-1 consequently with reduction in glutathione peroxidase in heart tissues. Furthermore, immunohistochemical examination of the heart tissues showed positive immuostaining for both 3-nitrotyrosine and caspase-3 with DNA-ladder formation in all diseased rats.
CONCLUSIONS:
11-keto BA in three dose levels exerted dose dependent cardioprotective effect manifested by dose-dependent reduction in serum lactate dehydrogenase and infract size through mechanisms related to enhancement of antioxidant capacity and prevention of inflammatory cascades. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)