| Structure Identification: |
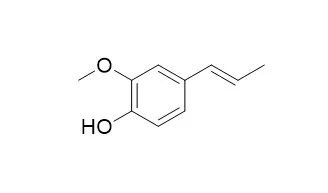
| Journal of japan oil chemists society, 1967, 16(6):344-347. | | Quantitative Analysis of the Mixture of Eugenol, cis- and trans-Isoeugenol by Gas-Liquid Chromatography with the Thermal Conductivity Detector.[Reference: WebLink] | This paper describes quantitative analysis of a mixture of eugenol, cis- and trans- isoeugenol(Isoeugenol,mixture of cis and trans) by gas-liquid chromatography with the thermal conductivity detector using silicone D.C. 550, D.O.S., Apiezone L., P.E.G. 1500 and 4000, and D.B.P. as the liquid substrates.
METHODS AND RESULTS:
The following results were observed.Every liquid substrate was used individually or in combinations of two or three. In the latter cases, the substrates were utilized in a mixed manner and in repeated coating with each of two or three substrates, resulting in the finding upon analysis that D.C. 550 and D.O.S. (mixed) gave excellent results.Twenty four samples of substrates were examined for retention time in the gas chromatograms in comparison with those of eugenol and cis- and trans- isomers of isoeugenol. Among them, carvone, acetyleugenol, diethyl phthalate, n-propyl cinnamate and showed different retention time as compared with those of homologues of eugenol. Thus these substrates indicated as usable, as the inner standard material in the quantitative analysis of gas chromatography.Upon analysis, acetyl eugenol showed migration of the acetyl group to isoeugenol in the sample, resulting in acetylisoeugenol. Carvone and n-propyl cinnamate were found as preferable rather than diethyl phthalate because of less tailing and shorter retention time in gas chromatograms. But carvone was apt to scale out in gas chromatograms even when used in low weight-ratios for a sample measured.Quantitative analysis of the sample prepared (eugenol : cis-isoeugenol : trans- isoeugenol=29.8:33. 8:36.4) was carried out within relative experimental errors of ±1.5%, using n-propyl cinnamate as an inner standard material.
CONCLUSIONS:
No transformation was seen between cis- and trans- isoeugenol in these analyzing procedures. | | Journal of Analytical & Applied Pyrolysis, 1990, 18(1):59-69. | | Analysis of lignin by pyrolysis-gas chromatography. I. Effect of inorganic substances on guaiacol-derivative yield from softwoods and their lignins.[Reference: WebLink] |
METHODS AND RESULTS:
To obtain lignin-derived pyrolysis products in larger yields, Curie-point pyrolysis was performed on the softwood species Japanese red pine (Pinus densiflora Sieb. et Zucc.), Japanese cedar (Cryptomeria japonica D. Don) and Japanese cypress (Chamaecyparis obtusa Endl.), and their dioxane lignins treated with various inorganic substances, over the temperature range 358-764.degree. C. The resulting guaiacol derivatives (guaiacol, 4-methyl-, 4-ethyl- and 4-vinylguaiacols, eugenol, vanillin, cis- and trans-isoeugenol(Isoeugenol,mixture of cis and trans), and acetovanillone) were determined by gas chromatography. The softwood samples sandwiched between two sheets of heat-resistant filter paper made of borosilicate glass fibers yielded from 1.56 to 1.64 times more guaiacol derivatives than unsandwiched samples. Sandwiching the dioxane lignins between heat-resistant papers also increased the guaiacol-derivative yield, by 13 to 27%.
CONCLUSIONS:
The sandwich-2 method developed in this study is widely applicable as a sample preparation method for increasing the yield of lignin-derived products on Curie-point pyrolysis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)