| Structure Identification: |
| BMC Genomics. 2011 Sep 29;12:475. | | Genome-wide examination of the transcriptional response to ecdysteroids 20-hydroxyecdysone and ponasterone A in Drosophila melanogaster.[Pubmed: 21958154] |
METHODS AND RESULTS:
Four ent-pimarene diterpenoids. ent-18-acetoxy-8(14)-pimarene-15S, 16-diol, ent-18-acetoxy-16-hydroxy-8(14)-pimaren-15-one, ent-16,18-dihydroxy-8(14)-pimaren-15-one and ent-19-nor-4,16,18-trihydroxy-8(14)-pimaren-15-one, together with three known damarane triterpenoids, Richenoic acid, eichleriainic acid and shoreic acid were isolated from the bark of Dysoxyhum hainanense Merr. Their structures were elucidated on the basis of spectroscopic techniques.
CONCLUSIONS:
The absolute configurations of four diterpenoids were assigned as ent-pimarene type by chemical transformation and by co-occurrence in the plant as well as by negative optical rotations for four compounds. | | Steroids. 2008 Dec 22;73(14):1452-64. | | Synthesis of ponasterone A derivatives with various steroid skeleton moieties and evaluation of their binding to the ecdysone receptor of Kc cells.[Pubmed: 18804484] |
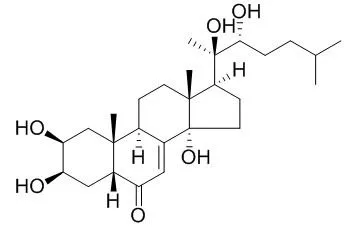
A series of Ponasterone A (PNA) derivatives with various steroid moieties were synthesized to measure their binding activity to the ecdysone receptors of Drosophila Kc cells.
METHODS AND RESULTS:
The activity of compounds was evaluated by determining the concentration required to give the 50% inhibition (IC(50) in M) of the incorporation of [(3)H]Ponasterone A to Drosophila Kc cells. Compounds with no functional groups such as OH and CO group in the steroid skeleton moiety were inactive. By the introduction of functional groups such as the OH and the CO group in the steroidal structure, these compounds became active. Some compounds containing the A/B-trans ring fusion, which is different from that (A/B-cis) of ecdysteroids were also active. The oxidation of CH(2) at 6-position to CO, enhanced the activity 19 times, but the activity was erased by the reduction of oxo to OH group at 6-position. The activity was enhanced about 250 times by the conversion of A/B ring configuration from trans [(20R,22R)-2beta,3beta,20,22-tetrahydroxy-5alpha-cholestan-6-one: pIC(50)=4.84] to cis [(20R,22R)-2beta,3beta,20,22-tetrahydroxy-5beta-cholestan-6-one: pIC(50)=7.23].
CONCLUSIONS:
The latter cis-type compound which is the most potent among compounds synthesized in this study was equipotent to the natural molting hormone, 20-hydroxyecdysone, even though it is 1/50 of Ponasterone A. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)