| In vivo: |
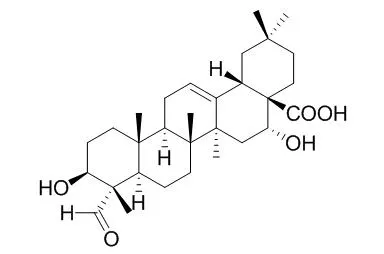
| J Ethnopharmacol. 2011 Jan 7;133(1):164-7. | | Antinociceptive activity of Quillaja saponaria Mol. saponin extract, quillaic acid and derivatives in mice.[Pubmed: 20951193] | Quillaja saponaria bark contains a high percentage of triterpene saponins and has been used for centuries as a cleansing and analgesic agent in Chilean folk medicine. The topical and systemic analgesic effects of a commercial partially purified saponin extract, 3β,16α-dihydroxy-23-oxoolean-12-en-28-oic acid (Quillaic acid), methyl 3β,16α-dihydroxy-23-oxoolean-12-en-28-oate and methyl 4-nor-3,16-dioxoolean-12-en-28-oate.
METHODS AND RESULTS:
The samples were assessed in mice using the topical tail-flick and i.p. hot-plate tests, respectively. RESULTS: All the samples showed activity in both analgesic tests in a dose-dependent manner. The most active against tail flick test was commercial partially purified saponin extract (EC50 27.9 mg%, w/v) and more than the ibuprofen sodium. On hot-plate test, methyl 4-nor-3, 16-dioxoolean-12-en-28-oate was the most active (ED50 12.2 mg/kg) and more than the ibuprofen sodium.
CONCLUSIONS:
The results of the present study demonstrated that Quillaja saponaria saponins, Quillaic acid, its methyl ester, and one of the oxidized derivatives of the latter, elicit dose-dependent antinociceptive effects in two murine thermal models. | | J Pharm Pharmacol. 2011 May;63(5):718-24. | | Topical anti-inflammatory activity of quillaic acid from Quillaja saponaria Mol. and some derivatives.[Pubmed: 21492174 ] | Quillaic acid is the major aglycone of the widely studied saponins of the Chilean indigenous tree Quillaja saponaria Mol. The industrial availability of quillaja saponins and the extensive functionalization of this triterpenoid provide unique opportunities for structural modification and pose a challenge from the standpoint of selectivity in regard to one or the other secondary alcohol group, the aldehyde, and the carboxylic acid functions. The anti-inflammatory activity of this sapogenin has not been studied previously and it has never been used to obtain potential anti-inflammatory derivatives.
METHODS AND RESULTS:
A series of Quillaic acid derivatives were prepared and subjected to topical assays for the inhibition of inflammation induced by arachidonic acid or phorbol ester.
CONCLUSIONS:
Quillaic acid exhibited strong topical anti-inflammatory activity in both models. Most of its derivatives were less potent, but the hydrazone 8 showed similar potency to Quillaic acid in the TPA assay.
METHODS:
A series of Quillaic acid derivatives were prepared and subjected to topical assays for the inhibition of inflammation induced by arachidonic acid or phorbol ester.
KEY FINDINGS:
Quillaic acid exhibited strong topical anti-inflammatory activity in both models. Most of its derivatives were less potent, but the hydrazone 8 showed similar potency to Quillaic acid in the TPA assay. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)