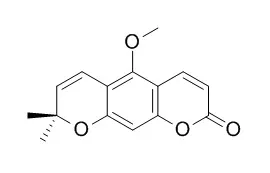
| Description: |
Xanthoxyletin shows potent antibacterial, fungicidal, and algicidal properties, it also has anticancer, and anti-inflammatory activities. It shows an inhibitory effect on iNOS protein expression at 10 microM, it also can inhibit the synthesis of nitric oxide and the protein expression of tumor necrosis factor-alpha and cyclooxygenase-2. Xanthoxyletin induces S phase arrest and apoptosis in human gastric adenocarcinoma SGC-7901 cells, the effects are associated with the DNA damage, apoptosis through mitochondrial dysfunction, and cell cycle arrest at S phase in a dose-dependent manner, it also can increase the production of reactive oxygen species. |
| In vitro: |
| Asian Pac J Cancer Prev. 2011;12(5):1219-23. | | Xanthoxyletin, a coumarin induces S phase arrest and apoptosis in human gastric adenocarcinoma SGC-7901 cells.[Pubmed: 21875271] | This study was conducted to explore the novel anticancer compounds from Chinese herbs. During the process of screening, to evaluate the potential chemopreventive effect of natural compounds, Xanthoxyletin was isolated from Erythrina variegata. It has been reported that Xanthoxyletin possesses antibacterial, fungicidal, and algicidal properties.
METHODS AND RESULTS:
In this study, we examined the antiproliferative effects of Xanthoxyletin against SGC-7901 cells and its ability to induce apoptosis and cell cycle arrest for the first time. We observed that its inhibitory effects on cells were associated with the DNA damage, apoptosis through mitochondrial dysfunction, and cell cycle arrest at S phase in a dose-dependent manner. Additionally, Xanthoxyletin also increased the production of reactive oxygen species in SGC-7901 cells.
CONCLUSIONS:
These results suggest that Xanthoxyletin may be promising anticancer agent and has worth for further mechanistic and therapeutic studies against gastric cancer. | | J Nat Med. 2009 Jan;63(1):21-7. | | Inhibitory effect of oxycoumarins isolated from the Thai medicinal plant Clausena guillauminii on the inflammation mediators, iNOS, TNF-alpha, and COX-2 expression in mouse macrophage RAW 264.7.[Pubmed: 18636311] |
METHODS AND RESULTS:
In the present study, we investigated the inhibitory effect of the known oxycoumarins poncitrin (3), osthol (4), and Xanthoxyletin (5), newly isolated from Clausena guillauminii (Rutaceae), together with the known carbazoles heptaphylline (1) and 7-methoxyheptaphylline (2) on inducible-nitric oxide synthase (iNOS) expression induced by lipopolysaccharide (LPS) and the NO generation in RAW 264.7 mouse macrophages. Isolation of active oxycoumarins was guided by Western blot analysis of iNOS protein expression. These oxycoumarins showed an inhibitory effect on iNOS protein expression at 10 microM. Further examination of the inhibitory effects of these compounds on inflammation mediators revealed that the synthesis of nitric oxide (NO) and the protein expression of tumor necrosis factor-alpha (TNF-alpha) and cyclooxygenase-2 (COX-2) were inhibited by 5.
CONCLUSIONS:
It was expected that these compounds show anti-inflammatory activities. | | Nat Prod Commun. 2010 Apr;5(4):559-61. | | Antimicrobial coumarins from the stem bark of Afraegle paniculata.[Pubmed: 20433072] |
METHODS AND RESULTS:
Eight compounds were isolated from the stem bark of the plant Afraegle paniculata. One of them, a dimethyl ether of S-trans-marmin (1), is reported as a new natural product. The structures were determined by comprehensive analyses of their 1D and 2D NMR spectroscopic and HREIMS data. The remaining seven known compounds, identified by comparing their spectroscopic data with those reported in the literature, were S-trans-marmin (2), psoralene (3), bergaptene (4), imperatorin (5), 2-(4-hydroxy-3,5-dimethoxyphenyl)-3-hydroxymethyl-2,3-dihydro- 1,4,5-trioxaphenanthren-6-one (6), Xanthoxyletin (7), and beta-sitosterol glucopyranoside.
CONCLUSIONS:
Preliminary studies indicated that compounds 2, 3, 5, and 7 showed potent antibacterial, fungicidal, and algicidal properties, while 6 showed only moderate algicidal property. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)