| In vitro: |
| Int J Mol Sci. 2013 Nov 13;14(11):22395-408. | | New benzo[c]phenanthridine and benzenoid derivatives, and other constituents from Zanthoxylum ailanthoides: Effects on neutrophil pro-inflammatory responses.[Pubmed: 24232457 ] |
METHODS AND RESULTS:
A new benzo[c]phenanthridine, oxynorchelerythrine (1), and two new benzenoid derivatives, methyl 4-(2-hydroxy-4-methoxy-3-methyl-4-oxobutoxy)benzoate (2) and (E)-methyl 4-(4-((Z)-3-methoxy-3-oxoprop-1-enyl)phenoxy)-2-methylbut-2-enoate (3), have been isolated from the twigs of Zanthoxylum ailanthoides, together with 11 known compounds (4-14). The structures of these new compounds were determined through spectroscopic and MS analyses.
CONCLUSIONS:
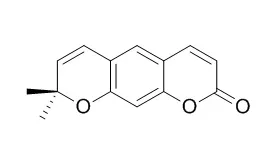
Among the isolated compounds, decarine (4), (-)-syringaresinol (6), (+)-episesamin (8), glaberide I (9), (-)-dihydrocubebin (10), and Xanthyletin (11) exhibited potent inhibition (IC50 values ≤ 4.79 μg/mL) of superoxide anion generation by human nutrophils in response to N-formyl-L-methionyl-L-leucyl-L-phenylalanine/cytochalasin B (fMLP/CB). Compounds 4, 8, and 11 also inhibited fMLP/CB-induced elastase release with IC50 values ≤ 5.48 μg/mL. | | Chem Pharm Bull (Tokyo). 2010 Jan;58(1):61-5. | | Anti-inflammatory principles from the stem and root barks of Citrus medica.[Pubmed: 20045968] |
METHODS AND RESULTS:
Bioassay-guided investigation of the anti-inflammatory principles from the stem and root barks of Citrus medica L. var. sarcodactylis SWINGLE has led to the isolation of a new coumarin, namely citrumedin-B (1) and thirty known compounds.
The anti-inflammatory components were Xanthyletin (2), nordentatin (3), atalantoflavon (4) and lonchocarpol A (5) which displayed potent nitric oxide (NO)-reducing activity in microglial cells. The structure of this new compound was completely elucidated using a combination of 2D NMR techniques (correlation spectroscopy (COSY), nuclear Overhauser effect spectroscopy (NOESY), heteronuclear multiple quantum coherence (HMQC) and heteronuclear multiple bond connectivity (HMBC)) and HR-electrospray ionization (ESI)-MS analyses. The known compounds were identified by comparison of their spectroscopic and physical data with those reported in the literature.
CONCLUSIONS:
These results can be inferred from the treatment of allergic response and inflammatory properties of Citrus medica L. var. sarcodactylis SWINGLE in traditional Chinese medicine. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)