Radix Polygoni Multiflori (RPM) and Radix Polygoni Multiflori Praeparata (RPMP) were traditionally widely used as Chinese herbal medicine. However, liver adverse reactions caused by RPM or RPMP were frequently reported all around the world recent years. The aim of this study was to study the cytotoxicities of RPM, RPMP and their major constituents on human liver cell L-02 simultaneously.
METHODS AND RESULTS:
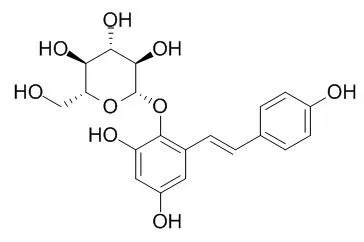
Multi-assays, including MTT assay, neutral red uptake (NRU) assay, LDH leakage percentage and liver enzyme secretion (AST, ALT and ALP) were used. Cytotoxicities of major chemical constituents of RPM, 2, 3, 5, 4'-tetrahydroxy-stilbene-2-O-β-D-glucoside (2,3,5,4'-Tetrahydroxyl diphenylethylene-2-O-glucoside,TSG), physcion and emodin, were tested. The cytotoxicities of water, 50% ethanol and 95% ethanol extractions of RPM and RPMP were tested. HPLC-DAD analysis was carried to reveal the content change of TSG, physcion and emodin after the processing procedure.
The TD(50) of TSG, physcion and emodin in MTT assay were >10,000 μM, 2853.61 μM and 520.37 μM. In the NRU assay, the TD(50) of TSG, physcion and emodin were much smaller (1401.53 μM, 1140.00 μM, and 3.80 μM). Emodin induced much severe liver enzyme secretion than TSG and physcion. Cell proliferation and LDH leakage rate showed no difference between RPM and RPMP extractions, but ALP, AST and ALT secretions in RPMP extractions were significant lower than that of PMR groups. Water extractions of RPM and RPMP were less toxic than any other solvent in most of the assays. Positive correlation was found between the TSG/emodin ratio and MTT survival rate. The emodin/physcion ratio also showed positive correlation with the LDH leakage percentage.
CONCLUSIONS:
In conclusion, Radix Polygonum multiflorum and Radix Polygonum multiflorum Praeparata were not liver injure inducing in our in vitro assays. However, the processing produce of RPM could reduce its effect on both cell proliferation and enzyme secretion of liver cell. Judging from cell proliferation, integrity of cell membrane and enzyme secretion, three major chemical constituents of RPM: TSG, physcion and emodin showed no, moderate and severe cytotoxicity against human liver cell line L-02 respectively. Chemical constituents-cytotoxicity relationship investigation revealed that TSG and physcion probably had attenuating effect to emodin. The attenuating mechanisms were still under investigation. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)