| Kinase Assay: |
| Apoptosis. 2010 Jun;15(6):715-27. | | Bilobalide prevents apoptosis through activation of the PI3K/Akt pathway in SH-SY5Y cells.[Pubmed: 20333467 ] | Bilobalide[(-)-Bilobalide], a sesquiterpene trilactone constituent of Ginkgo biloba leaf extracts, has been proposed to exert protective and trophic effects on neurons. However, mechanisms underlying the protective effects of bilobalide [(-)-Bilobalide]remain unclear. Using human SH-SY5Y neuroblastoma cells and primary hippocampal neurons, this study investigated the neuroprotective effects of bilobalide[(-)-Bilobalide].
METHODS AND RESULTS:
We mimicked aging-associated neuronal impairments by applying external factors (beta amyloid protein (Abeta) 1-42, H(2)O(2) and serum deprivation) consequently inducing cell apoptosis. As markers for apoptosis, cell viability, DNA fragmentation, mitochondrial membrane potential and levels of cleaved caspase 3 were measured. We found that, bilobalide[(-)-Bilobalide] prevented Abeta 1-42-, H(2)O(2)- and serum deprivation-induced apoptosis. To better understand the neuroprotective effects of bilobalide[(-)-Bilobalide], we also tested the ability of bilobalide[(-)-Bilobalide] to modulate pro-survival signaling pathways such as protein kinase C (PKC), extracellular-regulated kinase 1/2 (ERK1/2) and phosphatidylinositol 3-kinase (PI3K)/Akt pathways. It was found that, bilobalide[(-)-Bilobalide] dose-dependently increased PI3K activity and levels of phosphorylated Akt (p-Akt Ser473 and Thr308), which could be maintained up to at least 2 h after bilobalide withdrawal in cells treated with or without Abeta 1-42, H(2)O(2) or serum-free medium. In addition, application of PI3K/Akt inhibitor LY294002 could abrogate both the protective effects of bilobalide[(-)-Bilobalide] against Abeta 1-42-, H(2)O(2)- and serum deprivation-induced apoptotic cell damage and bilobalide[(-)-Bilobalide]-induced increase in PI3K activity and levels of p-Akt (Ser473 and Thr308). In contrast, application of PKC inhibitor staurosporine (STS) did not affect the protective effects of bilobalide[(-)-Bilobalide]. Moreover, no change in levels of phosphorylated ERK1/2 (p-ERK1/2) was observed in bilobalide-treated cells.
CONCLUSIONS:
These results further suggested that the PI3K/Akt pathway might be involved in the protective effects of bilobalide[(-)-Bilobalide]. Since modern technology allows production of purified bilobalide[(-)-Bilobalide] with high bioavailability, bilobalide[(-)-Bilobalide] may be useful in developing therapy for diseases involving age-associated neurodegeneration. |
|
| Cell Research: |
| Biosci Rep. 2011 Oct 1; 31(Pt 5): 439–447. | | Anti-ischaemic effects of bilobalide on neonatal rat cardiomyocytes and the involvement of the platelet-activating factor receptor[Pubmed: 21391918] | Terpene trilactones from Ginkgo biloba have been investigated extensively for their antioxidant and anti-ischaemic activities on the brain and the heart, but the mechanisms of these effects remain unclear.
METHODS AND RESULTS:
For the present study, a terpenoid constituent from G. biloba, bilobalide[(-)-Bilobalide], was screened for protective effects on the ischaemic heart and the involvement of the PAFR [PAF (platelet-activating factor) receptor] and the enzyme that degrades PAF, PAF-AH (PAF acetylhydrolase) during hypoxia. The PAF pathway is supposed to play a role in hypoxia and its regulation may prevent or alleviate MI (myocardial infarction). Cardiomyocytes from neonatal rat hearts were cultured and treated with different concentrations of bilobalide[(-)-Bilobalide] (500-0.5 ng/ml). After being subjected to a hypoxic environment, the cells' viability was evaluated and proteins as well as RNA were extracted for analysis by Western blotting and RT-PCR (reverse transcription PCR) respectively.
With the MI model we tested for bilobalide[(-)-Bilobalide]'s cardioprotective effects and the involvement of PAFR and PAF-AH. Bilobalide ((-)-Bilobalide,5 ng/ml) significantly decreased the mortality of cells in a concentration-dependent way. mRNA expression of PAFR was up-regulated in hypoxic cells but in the groups treated with bilobalide[(-)-Bilobalide], its expression was down-regulated to the level of the normal control. In hypoxic tissue, PAFR protein expression was also up-regulated, but was reduced in the bilobalide ((-)-Bilobalide,10 mg/kg of body weight) treated group.
CONCLUSIONS:
Our results indicate that PAF and its receptor may be involved in the cellular response of cardiomyocytes to hypoxia and that bilobalide may interact with this receptor to exert its cardioprotective effects. |
|
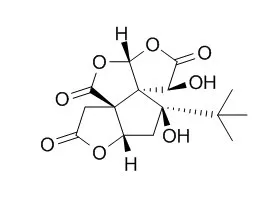
| Structure Identification: |
| The Korean Society of Pesticide Science,2001,5(1):24-9. | | Insecticidal Activities of Bilobalide from Ginkgo biloba Leaves and its Derivatives.[Reference: WebLink] | This study was conducted to investigate insecticidal activities of Ginkgo biloba (L.) leaves-derived bilobalide[(-)-Bilobalide] and its hydrolysis and oxidation products against adults of Nilaparavata lugens Stal.
METHODS AND RESULTS:
To find out active insecticidal moiety of bilobalide[(-)-Bilobalide], decomposed intermediates and derivatives of bilobalide[(-)-Bilobalide] were made by hydrolysis, oxidation, and acetylation. The structures of hydrolysis product by base and oxidation product by acid were identified as cyclopentenone analogues and trilactone sesquiterpene from dehydration of bilobalide[(-)-Bilobalide], respectively. Insecticidal activities of the decomposed intermediates and the derivatives of bilobalide[(-)-Bilobalide] decreased in the order of bilobalide[(-)-Bilobalide], monoacetate, ginkgolide C, oxidation product, diacetate, and hydrolysis product.
CONCLUSIONS:
Therefore, trilactone structure of bilobalide[(-)-Bilobalide] may be essential for its insecticidal activity. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)