| In vitro: |
| Sci Rep. 2015 Aug 6;5:12889. | | Inhibition of protein kinase C by isojacareubin suppresses hepatocellular carcinoma metastasis and induces apoptosis in vitro and in vivo.[Pubmed: 26245668 ] | Targeted inhibition of protein kinase C (PKC) inhibits hepatocellular carcinoma (HCC) proliferation and metastasis. We previously reported the cytotoxicity of a series of synthetic phenyl-substituted polyoxygenated xanthone derivatives against human HCC.
METHODS AND RESULTS:
In the current study, the most potent natural product, Isojacareubin (ISJ), was synthesized, and its cellular-level antihepatoma activities were evaluated. ISJ significantly inhibited cell proliferation and was highly selective for HCC cells in comparison to nonmalignant QSG-7701 hepatocytes. Moreover, ISJ exhibited pro-apoptotic effects on HepG2 hepatoma cells, as well as impaired HepG2 cell migration and invasion. Furthermore, ISJ was a potent inhibitor of PKC, with differential actions against various PKC isotypes. ISJ selectively inhibited the expression of aPKC (PKCζ) in the cytosol and the translocation of cytosolic PKCζ to membrane site. ISJ also directly interacted with cPKC (PKCα) and nPKC (PKCδ, PKCε and PKCμ) and thereby inhibited the early response of major MAPK phosphorylation and the late response of HCC cell invasion and proliferation. In a hepatoma xenograft model, ISJ pretreatment resulted in significant antihepatoma activity in vivo.
CONCLUSIONS:
These findings identify ISJ as a promising lead compound for the development of new antihepatoma agents and may guide the search for additional selective PKC inhibitors. | | Arch Pharm Res. 2014 Aug;37(8):972-7. | | Anti-Helicobacter pylori xanthones of Garcinia fusca.[Pubmed: 24155023 ] |
METHODS AND RESULTS:
A new geranylated xanthone derivative, fuscaxanthone I (1), along with nine xanthones (2-9 and 11), a biphenyl (10) and three biflavonoids (12-14) were isolated from the roots of Garcinia fusca Pierre. Compounds 8, 10 and 11-14 were reported from this plant species for the first time. Their structures were elucidated by spectroscopic analyses, including 1D- and 2D-NMR and MS. The isolated compounds were evaluated for antibacterial activity against Helicobacter pylori.
CONCLUSIONS:
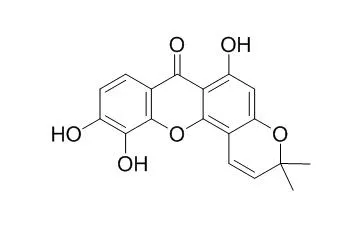
Cowaxanthone (5) and fukugiside (14) exhibited stronger inhibitory activity against H. pylori DMST reference strain at MICs 4.6 and 10.8 μM, respectively, than that of the control metronidazole. Isojacareubin (8) displayed the most potent activity against H. pylori HP40 clinical isolate with MIC 23.9 μM, which was approximately two times greater than that of the standard drug amoxicillin. | | Int J Mol Sci. 2012;13(7):8210-8. | | Isojacareubin from the Chinese herb Hypericum japonicum: potent antibacterial and synergistic effects on clinical methicillin-resistant Staphylococcus aureus (MRSA).[Pubmed: 22942699 ] |
METHODS AND RESULTS:
Through bioassay-guided fractionation of the extracts from the aerial parts of the Chinese herb Hypericum japonicum Thunb. Murray, Isojacareubin (ISJ) was characterized as a potent antibacterial compound against the clinical methicillin-resistant Staphylococcus aureus (MRSA). The broth microdilution assay was used to determine the minimal inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) of ISJ alone. The results showed that its MICs/MBCs ranged from 4/16 to 16/64 μg/mL, with the concentrations required to inhibit or kill 50% of the strains (MIC(50)/MBC(50)) at 8/16 μg/mL. Synergistic evaluations of this compound with four conventional antibacterial agents representing different types were performed by the chequerboard and time-kill tests. The chequerboard method showed significant synergy effects when ISJ was combined with Ceftazidime (CAZ), Levofloxacin (LEV) and Ampicillin (AMP), with the values of 50% of the fractional inhibitory concentration indices (FICI(50)) at 0.25, 0.37 and 0.37, respectively. Combined bactericidal activities were also observed in the time-kill dynamic assay.
CONCLUSIONS:
The results showed the ability of ISJ to reduce MRSA viable counts by log(10)CFU/mL at 24 h of incubation at a concentration of 1 × MIC were 1.5 (LEV, additivity), 0.92 (CAZ, indifference) and 0.82 (AMP, indifference), respectively. These in vitro anti-MRSA activities of ISJ alone and its synergy with conventional antibacterial agents demonstrated that ISJ enhanced their efficacy, which is of potential use for single and combinatory therapy of patients infected with MRSA.
|
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)