| Structure Identification: |
| Acs Earth & Space Chemistry, 2019, 3(4):571-580. | | Insights into the Headgroup and Chain Length Dependence of Surface Characteristics of Organic-Coated Sea Spray Aerosols.[Reference: WebLink] | The structure of sea spray aerosols (SSAs) has been described as a saline core coated by organic surfactants. The presence of surface-active compounds at the interface can exert significant effect on physical, chemical, and optical properties of SSAs.
METHODS AND RESULTS:
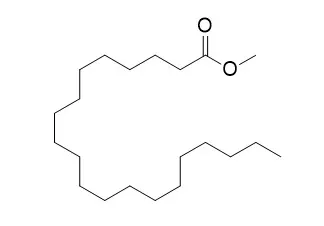
The surfactant molecules chosen for this study, palmitic acid (PA), stearic acid (SA), arachidic acid (AA), methyl palmitate (MP), methyl stearate (MS), and Methyl arachidate (MA), were used to explore the effect of alkyl chain length, headgroups, and sea salts on the surface properties of these monolayers. Surface pressure–area (π–A) isotherms obtained with a Langmuir trough was used for revealing macroscopic phase behavior of the monolayers. Moreover, infrared reflection absorption spectroscopy (IRRAS) was employed to investigate the interfacial organization at the molecular level. The π–A isotherms indicated that sea salts, present in the subphase, have an intense condensing effect on fatty acid monolayers while exerting an expanding effect on fatty acid methyl ester monolayers, which was confirmed by results from IRRAS experiments. IRRAS further uncovered the all-trans conformation of the hydrocarbon chains, which can be evidenced by the relatively low νa(CH2) and νs(CH2) stretching vibrations. The conformational order changes in the alkyl chains of different film-forming species (C16 < C18 < C20) were directly revealed by analyzing the intensity ratios of the νa(CH2) and νs(CH2) bands.
CONCLUSIONS:
Thus, all three factors alter the phase behavior and molecular packing of the monolayers at the air–aqueous interface. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)