| In vitro: |
| J Chem Ecol. 1993 Oct;19(10):2279-84. | | Herbicidal activity of sulforaphene from stock (Matthiola incana).[Pubmed: 24248575] |
METHODS AND RESULTS:
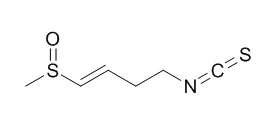
A herbicidal compound was isolated from extracts ofMatthiola incana and identified as Sulforaphene (4-methylsulfinyl-3-butenyl isothiocyanate).
CONCLUSIONS:
The ED50 of this compound against velvetleaf seedlings was approximately 2×10(-4) M. Glucoraphenin, the glucosinolate that is the natural precursor of Sulforaphene, was less phytotoxic, with an ED50 of near 6×10(-3)M. | | Breast Cancer Res Treat. 2016 Jul;158(2):277-86. | | Sulforaphene inhibits triple negative breast cancer through activating tumor suppressor Egr1.[Pubmed: 27377973 ] | Sulforaphene (SFE, 4-methylsufinyl-3-butenyl isothiocyanate) is a member of isothiocyanates, which is derived from radish seeds. It has shown that multiple isothiocyanates, such as sulforaphane, can effectively inhibit cancer cell proliferation in vitro and in vivo. However, it is still largely unknown if SFE could impact breast cancer.
METHODS AND RESULTS:
In this study, we investigated the anticancer effects of SFE on triple negative breast cancer (TNBC) via a series of in vitro and in vivo assays. We found that SFE can significantly inhibit cell proliferation in multiple TNBC cell lines through inducing G2/M phase arrest as well as cell apoptosis. Nude mice xenograft assays support the anti-TNBC role of SFE in vivo. Interestingly, SFE can repress expression of cyclinB1, Cdc2, and phosphorylated Cdc2, and, then, induced G2/M phase arrest of TNBC cells. To identify SFE target genes, we detected genome-wide gene expression changes through gene expression profiling and observed 27 upregulated and 18 downregulated genes in MDA-MB-453 cells treated with SFE. Among these genes, Egr1 was successfully validated as a consistently activated gene after SFE treatment in TNBC MDA-MB-453 and MDA-MB-436 cells. Egr1 overexpression inhibited proliferation of TNBC cells. However, Egr1 knockdown using siRNAs significantly promoted TNBC cell growth, indicating the tumor suppressor nature of Egr1.
CONCLUSIONS:
In sum, we for the first time found that SFE might be a potential anti-TNBC natural compound and its antiproliferation effects might be mediated by tumor suppressor Egr1. | | Ashs Conference. 2013. | | Characterization of Anti-proliferative and Antibacterial Properties of Sulforaphene Obtained from Radish Seeds,[Reference: WebLink] |
Many isothiocyanates (ITCs), are a mainly hydrolysis product in glucosinolates (GSLs), have been demonstrated the noteworthy overcoming impact against the survival and proliferation of cancer cells and their modulation of apoptosis and cell cycle progression by numerous molecular basis studies (Zang et al., 2006) , such as sulforaphane (SFA) isolated from broccoli seed and sprouts. By the way, Sulforaphene (SFE), is a major ITCs in radish seed, have been reported the potency of biological activity, a little bit recently. On the other hands, while much researches were known that SFA in broccoli has the excellent anticancer effects such as induction of apoptosis and detoxification enzymes in vitro and in vivo (Fahey et al., 2002), SFE in radish was hardly the biological study in spite of their similar chemical structure in comparison with SFA.
METHODS AND RESULTS:
In the present study, I demonstrated the broadly biological activity of SFE against cancer cells, Helicobacter pylori (H. pylori) and multi-drug resistance pathogens. In 4 cancer cells isolated from each four organisms were notably inhibited the proliferation treated with purified SFE (IC50 = 10.0–23 μg/mL). I also characterized that SFE modulated an induction of apoptosis pathway against A549 cancer cell through the proteins expressions related with apoptosis pathway. In addition, the highly bacteriocidal potency (MIC90 = 0.6–5.0 μg/mL) of SFE was exhibited against H. pylori, particularly antibiotic resistant strain (212 strain, MIC90 = 0.6 μg/mL). MRSA (Methicillin-resistant staphylococcus aureus), is known as super bacteria, also were inhibited by SFE (MIC90 = 10–20 μg/mL), whereas the MIC 90 value of MSSA (Methicillin-susceptible staphylococcus aureus) by SFE had little significant.
CONCLUSIONS:
These results suggested that the antibiotic potency of SFE in radish seeds would be associated with the potency in a broad range of cancer cells and antibiotic resistant pathogens. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)