| In vitro: |
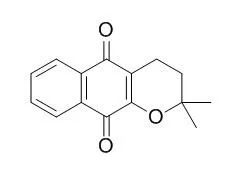
| J Org Chem. 2015 Aug 7;80(15):7340-50. | | Photoconversion of β-Lapachone to α-Lapachone via a Protonation-Assisted Singlet Excited State Pathway in Aqueous Solution: A Time-Resolved Spectroscopic Study.[Pubmed: 26133974 ] |
METHODS AND RESULTS:
The photophysical and photochemical reactions of β-lapachone were studied using femtosecond transient absorption, nanosecond transient absorption, and nanosecond time-resolved resonance Raman spectroscopy techniques and density functional theory calculations. In acetonitrile, β-lapachone underwent an efficient intersystem crossing to form the triplet state of β-lapachone. However, in water-rich solutions, the singlet state of β-lapachone was predominantly quenched by the photoinduced protonation of the carbonyl group at the β position (O9). After protonation, a series of fast reaction steps occurred to eventually generate the triplet state α-lapachone intermediate. This triplet state of α-lapachone then underwent intersystem crossing to produce the ground singlet state of α-lapachone as the final product. 1,2-Naphthoquinone is examined in acetonitrile and water solutions in order to elucidate the important roles that water and the pyran ring play during the photoconversion from β-lapachone to α-lapachone. β-Lapachone can also be converted to α-lapachone in the ground state when a strong acid is added to an aqueous solution.
CONCLUSIONS:
Our investigation indicates that β-lapachone can be converted to α-lapachone by photoconversion in aqueous solutions by a protonation-assisted singlet excited state reaction or by an acid-assisted ground state reaction. | | Invest New Drugs. 2010 Apr;28(2):139-44. | | Comparison of the cytotoxic effect of lapachol, alpha-lapachone and pentacyclic 1,4-naphthoquinones on human leukemic cells.[Pubmed: 19255723] |
METHODS AND RESULTS:
The pentacyclic 1,4-naphthoquinones 1a-d were cytotoxic (IC(50) approximately 2-7 microM) to human leukemic cell lines K562 (oxidative stress-resistant), Lucena-1 (MDR phenotype) and Daudi. Fresh leukemic cells obtained from patients, some with the MDR phenotype, were also sensitive to these compounds. The pentacyclic 1,4-naphthoquinones 1a and 1c induced apoptotic cell death in cells from leukemic patients as determined by flow cytometry. Conversely, the cell lines were highly insensitive to lapachol (2) and alpha-Lapachone (3). Mitomycin-C inhibited cell proliferation at concentrations as low as 0.5 microM. The low toxicity against lymphocytes activated by phytohemagglutinin shows that these compounds are selective for the cancer cells studied.
CONCLUSIONS:
Previous data suggest that these compounds (1a-d) can be bioactivated in situ by reduction followed by rearrangement leading to enones, which are powerful alkylating agents. In contrast, lapachol (2) and beta-lapachone (3), which cannot be bioactivated by reduction, showed little activity against the same cell lines. | | Parasitol Res. 2006 Sep;99(4):429-33. | | Oxyrane derivative of alpha-lapachone is potent growth inhibitor of Trypanosoma cruzi epimastigote forms.[Pubmed: 16596415 ] |
METHODS AND RESULTS:
The investigation of trypanocidal effects against Trypanosoma cruzi and cytotoxicity in VERO cell line of several oxyranes structurally related to beta-lapachone, nor-beta-lapachone, alpha-Lapachone, and 4-methoxy-1,2-naphthoquinone is described.
CONCLUSIONS:
It was found that the oxyranes 10 derived from alpha-Lapachone showed an approximately the same trypanocidal activity of beta-lapachone. In addition, all the oxyranes showed less cytotoxicity than the corresponding naphthoquinones. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)