Natural Products
alpha-Hederin
| Catalog No. | CFN98325 | 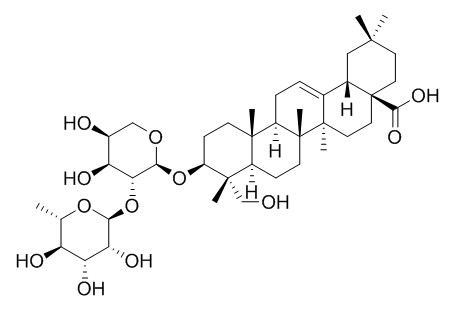 |
| CAS No. | 27013-91-8 | |
| Molecular Weight: | 751.0 | |
| Molecular Formula | C41H66O12 | |
| DBs | [PubChem]:274952095 [ChEMBL]: [PCIDB]:3528 |
Standard InChI:
InChI=1S/C41H66O12/c1-21-28(44)30(46)31(47)33(51-21)53-32-29(45)24(43)19-50-34(32)52-27-11-12-37(4)25(38(27,5)20-42)10-13-40(7)26(37)9-8-22-23-18-36(2,3)14-16-41(23,35(48)49)17-15-39(22,40)6/h8,21,23-34,42-47H,9-20H2,1-7H3,(H,48,49)/t21?,23-,24-,25+,26+,27-,28-,29-,30-,31?,32?,33-,34-,37-,38-,39+,40+,41-/m0/s1
Biological Activity
Alpha-hederin as a pro-drug, has cytotoxic and apoptotic/necrotic effects in a dose- and time-dependent manner on on four human cancer cell lines [A549 (lung carcinoma), HEp-2 (larynx epidermoid carcinoma), HT-29 (colon adenocarcinoma) and MIA PaCa-2 (pancreas carcinoma)] .[1]
Alpha-hederin is a saponin isolated from ivy, has anti-oxidant activity and acute anti-inflammatory activity in carrageenan-induced rat paw edema.[2]
Alpha-hederin, chlorophyllin and ascorbic acid have protective effects towards the induction of micronuclei by doxorubicin in cultured human lymphocytes.[3]
Alpha-hederin has antileishmanial activity ,exhibits a strong antiproliferative activity on all stages of development of the parasite by altering membrane integrity and potential in Leishmania .[4]
Alpha-hederin affects the binding behavior, dynamics, and regulation of beta 2-adrenergic receptors.[5]
Alpha-Hederin can increase isoprenaline-induced relaxation indirectly, probably by inhibiting heterologous desensitization induced by high concentrations of muscarinic ligands like .[6]
Alpha-hederin at sub-IC(50) cytotoxic concentrations enhances 5-fluorouracil (5-FU) cytotoxicity about 3.3-fold, suggests that it is possible to optimize colorectal cancer cell sensitivity to 5-FU with alpha-hederin.[7]
Alpha-hederin has anticancer effect on breast cancer cells.[8]
Product
References
[1] Rooney S, Ryan M F. Anticancer Res, 2005, 25(3B):2199-204.
[2] Gepdiremen A, Mshvildadze V, S체leyman H, et al. Phytomedicine, 2005, 12(6-7):440-4.
[3] Gülçin I, Mshvildadze V, Gepdiremen A,et al. Planta Med, 2004, 70(6):561-3.
[4] Amara-Mokrane Y A, Lehucher-Michel M P, Balansard G, et al. Mutagenesis, 1996, 11(2):161-7.
[5] Delmas F, Di G C R, Gasquet M, et al. Planta Med, 2000, 66(4):343-7.
[6] Sieben A, Prenner L, Sorkalla T, et al. Biochemistry, 2009, 48(15):3477-82.
[7] Anne Wolf, Reinoud Gosens, Herman Meurs, et al. Phytother Res Ptr, 2008, 22(10):1299-302.
[8] Cheng L, Xia T S, Wang Y F, et al. Int J Oncol, 2014, 45(2):757-63.
[9] Li Y, Li J, Huang J, et al. Chinese Agr Sci Bull, 2014, 30(13):190-3.
Product Use Citation





