| In vitro: |
| Planta Med. 2011 Jan;77(1):77-80. | | Leishmanicidal and reversal multidrug resistance constituents from Aeonium lindleyi.[Pubmed: 20665372 ] |
METHODS AND RESULTS:
A new bicyclic diterpene with a labdane skeleton, 7-oxo-labd-8-en-15-ol ( 1), along with two known diterpenes and ten flavonoids were isolated from the leaves of Aeonium lindleyi (Crassulaceae). Their structures were elucidated on the basis of spectroscopic data, including 1D and 2D NMR experiments, and comparison with spectroscopic data reported in the literature.
CONCLUSIONS:
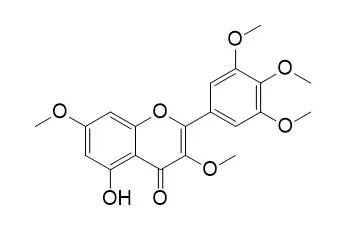
Labdan-8 α,15-diol (2) and labd-8(17)-en-3 β,15-diol (3) showed leishmanicidal activity against Leishmania tropica (IC (50) = 77.0 µM) and Leishmania braziliensis (IC (50) = 68.0 µM) similar to ketoconazole used as positive control. 5,3'-Dihydroxy-3,7,4',5'-tetramethoxyflavone (8) and Combretol (9) showed moderate activity (growth inhibition 87.3 and 73.0 %, respectively, at 50 µM) against a multidrug-resistant L. tropica line. | | J Nat Prod. 2006 Feb;69(2):287-9. | | Cytotoxic diterpenes from Cassipourea madagascariensis from the Madagascar rainforest.[Pubmed: 16499334 ] |
METHODS AND RESULTS:
Bioassay-directed fractionation of ethanol extracts of the roots and leaves of the plant Cassipourea madagascariensis resulted in the isolation of the two new terpenoids cassipourol (1) and cassipouryl acetate (2) in addition to the three known compounds, 3beta,30-dihydroxylup-20(29)-ene (3), 30-hydroxylup-20(29)-en-3-one (4), and Combretol (5). The structures of the two new compounds were established on the basis of 1D and 2D NMR spectroscopic data and chemical conversion.
CONCLUSIONS:
All the isolated compounds were tested against the A2780 human ovarian cancer cell line; the two diterpenes (1 and 2) showed moderate cytotoxic activity, while the three known compounds (3-5) were weakly active. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)