| Cell Research: |
| Int J Oncol. 2011 Mar;38(3):761-7. | | Ganodermanontriol, a lanostanoid triterpene from Ganoderma lucidum, suppresses growth of colon cancer cells through ß-catenin signaling.[Pubmed: 21225227] | Colorectal cancer is one of the most common cancers in men and women in the world. Previous molecular studies have revealed that deregulation of the ß-catenin signaling pathway plays a crucial role in the progression of colorectal cancer. Therefore, modulation of the ß-catenin pathway offers a strategy to control colorectal cancer progression. The medicinal mushroom Ganoderma lucidum (GL) is a rich source of triterpenes with anticancer properties.
METHODS AND RESULTS:
Here, we show that Ganodermanontriol (GNDT), a purified triterpene from GL, inhibited proliferation of HCT-116 and HT-29 colon cancer cells without a significant effect on cell viability. Moreover, GNDT inhibited transcriptional activity of ß-catenin and protein expression of its target gene cyclin D1 in a dose-dependent manner. A marked inhibition effect was also seen on Cdk-4 and PCNA expression, whereas expression of Cdk-2, p21 and cyclin E was not affected by the GNDT treatment. In addition, GNDT caused a dose-dependent increase in protein expression of E-cadherin and ß-catenin in HT-29 cells. Finally, GNDT suppressed tumor growth in a xenograft model of human colon adenocarcinoma cells HT-29 implanted in nude mice without any side-effects and inhibited expression of cyclin D1 in tumors.
CONCLUSIONS:
In conclusion, our data suggest that Ganodermanontriol might be a potential chemotherapeutic agent for the treatment of cancer. |
|
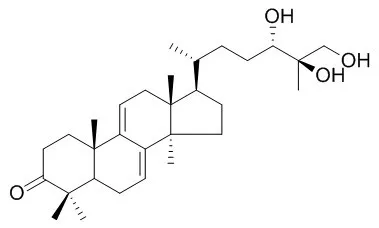
| Structure Identification: |
| Phytochemistry. 1998 Nov;49(6):1651-7. | | Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum.[Pubmed: 9862140] | A new highly oxygenated triterpene named ganoderic acid alpha has been isolated from a methanol extract of the fruiting bodies of Ganoderma lucidum together with twelve known compounds.
METHODS AND RESULTS:
The structures of the isolated compounds were determined by spectroscopic means including 2D-NMR. Ganoderiol F and Ganodermanontriol were found to be active as anti-HIV-1 agents with an inhibitory concentration of 7.8 micrograms ml-1 for both, and ganoderic acid B, ganoderiol B, ganoderic acid C1, 3 beta-5 alpha-dihydroxy-6 beta-methoxyergosta-7,22-diene, ganoderic acid alpha, ganoderic acid H and ganoderiol A were moderately active inhibitors against HIV-1 PR with a 50% inhibitory concentration of 0.17-0.23 mM. | | Planta Med. 2001 Dec;67(9):811-4. | | Anticomplement activity of terpenoids from the spores of Ganoderma lucidum.[Pubmed: 11745016 ] | A new lanostane-type terpenoid, lucidenic acid SP1 (1), was isolated from a CHCl(3)-soluble fraction of Ganoderma lucidum spores together with four other known compounds (2 - 5).
METHODS AND RESULTS:
The structure of lucidenic acid SP1 was determined to be 3 beta,7 beta-dihydroxy-4,4,14 alpha-trimethyl-11,15-dioxo-5 alpha-chol-8-en-24-oic acid by spectroscopic means including 2D-NMR. Twelve triterpenes (1-12) isolated from G. lucidum spores were investigated in vitro for their anticomplementary activity. Compounds 1 - 5 were inactive, whereas ganoderiol F (8), ganodermanondiol (9) and Ganodermanontriol (10) showed a strong anticomplement activity against the classical pathway (CP) of the complement system with IC(50) values of 4.8, 41.7, and 17.2 microM, respectively. The potency of these triterpene alcohols (8-10) in inhibiting CP activity was improved when the number of hydroxymethyl groups on the side chain moiety is increased. On the other hand, the ganoderic acids 1-7, which contain a carboxyl group in the side chain, and lucidumols A and B (11, 12) had little activity on this system. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)