| Description: |
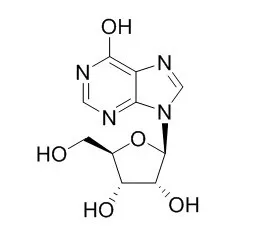
Inosine, an endogenous purine nucleoside, has immunomodulatory, neuroprotective, and analgesic properties.Inosine is a cardiotonic agent, can treat cardiac disorders.Inosine can to be capable of forming base pairs with Adenine HFN72-P, Cytosine HDR44-O or Uracil BTP40-U thus contributing to genetic code degeneracy by causing stable mispairings. |
| In vivo: |
| JAMA Neurol. 2014 Feb;71(2):141-50. | | Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial.[Pubmed: 24366103] | Convergent biological, epidemiological, and clinical data identified urate elevation as a candidate strategy for slowing disability progression in Parkinson disease (PD).
To determine the safety, tolerability, and urate-elevating capability of the urate precursor Inosine in early PD and to assess its suitability and potential design features for a disease-modification trial.
METHODS AND RESULTS:
The Safety of Urate Elevation in PD (SURE-PD) study, a randomized, double-blind, placebo-controlled, dose-ranging trial of Inosine, enrolled participants from 2009 to 2011 and followed them for up to 25 months at outpatient visits to 17 credentialed clinical study sites of the Parkinson Study Group across the United States. Seventy-five consenting adults (mean age, 62 years; 55% women) with early PD not yet requiring symptomatic treatment and a serum urate concentration less than 6 mg/dL (the approximate population median) were enrolled.
Participants were randomized to 1 of 3 treatment arms: placebo or Inosine titrated to produce mild (6.1-7.0 mg/dL) or moderate (7.1-8.0 mg/dL) serum urate elevation using 500-mg capsules taken orally up to 2 capsules 3 times per day. They were followed for up to 24 months (median, 18 months) while receiving the study drug plus 1 washout month.
The prespecified primary outcomes were absence of unacceptable serious adverse events (safety), continued treatment without adverse event requiring dose reduction (tolerability), and elevation of urate assessed serially in serum and once (at 3 months) in cerebrospinal fluid. RESULTS Serious adverse events (17), including infrequent cardiovascular events, occurred at the same or lower rates in the Inosine groups relative to placebo. No participant developed gout and 3 receiving Inosine developed symptomatic urolithiasis. Treatment was tolerated by 95% of participants at 6 months, and no participant withdrew because of an adverse event. Serum urate rose by 2.3 and 3.0 mg/dL in the 2 Inosine groups (P < .001 for each) vs placebo, and cerebrospinal fluid urate level was greater in both Inosine groups (P = .006 and <.001, respectively). Secondary analyses demonstrated nonfutility of Inosine treatment for slowing disability.
CONCLUSIONS:
Inosine was generally safe, tolerable, and effective in raising serum and cerebrospinal fluid urate levels in early PD. The findings support advancing to more definitive development of Inosine as a potential disease-modifying therapy for PD. | | Brain Res. 2014 Mar 25;1555:78-88. | | Inosine improves functional recovery after experimental traumatic brain injury.[Pubmed: 24502983] | Despite years of research, no effective therapy is yet available for the treatment of traumatic brain injury (TBI). The most prevalent and debilitating features in survivors of TBI are cognitive deficits and motor dysfunction. A potential therapeutic method for improving the function of patients following TBI would be to restore, at least in part, plasticity to the CNS in a controlled way that would allow for the formation of compensatory circuits. Inosine, a naturally occurring purine nucleoside, has been shown to promote axon collateral growth in the corticospinal tract (CST) following stroke and focal TBI.
METHODS AND RESULTS:
In the present study, we investigated the effects of Inosine on motor and cognitive deficits, CST sprouting, and expression of synaptic proteins in an experimental model of closed head injury (CHI). Treatment with Inosine (100 mg/kg i.p. at 1, 24 and 48 h following CHI) improved outcome after TBI, significantly decreasing the neurological severity score (NSS, p<0.04 vs. saline), an aggregate measure of performance on several tasks. It improved non-spatial cognitive performance (object recognition, p<0.016 vs. saline) but had little effect on sensorimotor coordination (rotarod) and spatial cognitive functions (Y-maze). Inosine did not affect CST sprouting in the lumbar spinal cord but did restore levels of the growth-associated protein GAP-43 in the hippocampus, though not in the cerebral cortex.
CONCLUSIONS:
Our results suggest that Inosine may improve functional outcome after TBI. | | Transplant Proc. 2014 Jan-Feb;46(1):40-5. | | Preconditioning with gabexate is superior to inosine for ameliorating acute renal ischemia-reperfusion injury in rats.[Pubmed: 24507023] | The objective of this study was to compare the protease inhibitor gabexate with widely used Inosine for reducing renal ischemia-reperfusion injury.
METHODS AND RESULTS:
A total of 48 rats were divided into 4 groups of 12 and administered gabexate, Inosine, normal saline (NS), or nothing by injection through the vena dorsalis of the penis. Then all rats were subjected to right nephrectomy and 30-minute warm ischemia of the left kidney. At 24 and 48 hours after reperfusion, blood samples were collected from the inferior vena cava and serum creatinine (SCr) was assayed. Left kidney tissue was homogenized and used to assay malondialdehyde (MDA) and superoxide dismutase (SOD). The tissue was also analyzed using hematoxylin-eosin (HE) staining, TUNEL staining, and NF-κB immunohistochemistry.
SCr level decreased after reperfusion more in the gabexate group than in the other groups. Reperfused kidney tissue in the gabexate group showed lower MDA levels but higher SOD activity than did tissue in the Inosine and saline groups, as well as lower pathology scores based on HE staining, lower necrosis index, and lower levels of NF-κB expression (all P < .05). Tissue in the Inosine and saline groups showed similar necrosis index and NF-κB expression (P > .05).
CONCLUSIONS:
Preconditioning with gabexate is superior to preconditioning with Inosine for ameliorating rat renal ischemia-reperfusion injury. Future studies are needed to verify the effects of gabexate in the clinic, especially for kidney transplantation. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)