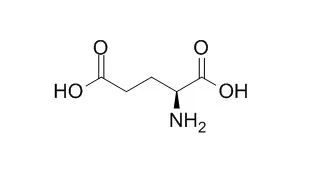
| Structure Identification: |
| Nature, 1974 , 248 (5451) :804-5. | | Spinal interneurone excitation by conformationally restricted analogues of L-glutamic acid[Reference: WebLink] | L-Glutamic acid is probably an excitatory transmitter of major significance in the mammalian central nervous system1.
METHODS AND RESULTS:
The L-Glutamic acid molecule is relatively flexible, and in an attempt to gain some insight into its possible shape(s) during activation of receptors associated with excitation of central neurones, a study was made of four conformationally restricted analogues: (±)-cis-1-aminocyclohexane-1,3-dicarboxylic acid (‘cyclo-glutamic’ acid), ibotenic acid, kainic acid and its dihydro derivative. | | Bioconjug. Chem., 1999 , 10 (1) :137-40. | | L-Glutamic acid and L-lysine as useful building blocks for the preparation of bifunctional DTPA-like ligands.[Reference: WebLink] |
METHODS AND RESULTS:
Bisalkylation of suitably protected L-Glutamic acid and L-lysine derivatives with tert-butyl N-(2-bromoethyl)iminodiacetate 2, followed by deprotection of the omega functional group affords N, N-bis[2-[bis[2-(1, 1-dimethylethoxy)-2-oxoethyl]amino]ethyl]-L-Glutamic acid 1-(1, 1-dimethylethyl) ester 4 and N2,N2-bis[2-[bis[2-(1, 1-dimethylethoxy)-2-oxoethyl]amino]ethyl]-L-lysine 1,1-dimethylethyl ester 7. Such compounds feature a carboxylic or an amino group, respectively, which are available for conjugation with a suitable partner via formation of an amide bond. The conjugates, which can be prepared in this way, contain a chelating subunit in which all five acetic residues of DTPA are available for the complexation of metal ions. Direct bisalkylation of glycine with 2 promptly gives N, N-bis[2-[bis[2-(1,1-dimethylethoxy)-2-oxoethyl]amino]ethyl]glycine 11.
CONCLUSIONS:
The latter allows to achieve conjugates in which the central acetic group of DTPA is selectively converted into an acetamide. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)