| Structure Identification: |
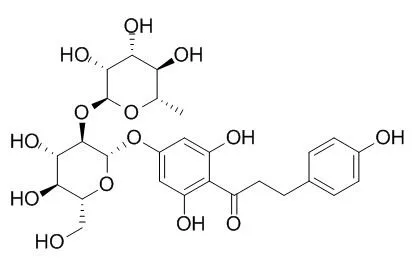
| 《Fine Chemical Intermediates》 2011-04 | | Study on Preparation of Naringin Dihydrochalcone[Reference: WebLink] | | The process for preparation of Naringin dihydrochalcone from naringin was investigated.The product was obtained in 90% yield under optimum conditions of pH 13,temperature 45℃,H2 pressure 0.5 MPa,raney nickel loading 5%,and reaction time 4 h. | | Food Research International Volume 54, Issue 1, November 2013, Pages 691–696 | | Preparation and physicochemical characterization of the supramolecular inclusion complex of naringin dihydrochalcone and hydroxypropyl-β-cyclodextrin[Reference: WebLink] | Naringin dihydrochalcone (naringin DC) is an intense sweetener and a strong antioxidant with potential applications in many food and pharmaceutical products. However, its low water solubility impedes the realization of these applications.
METHODS AND RESULTS:
This study investigated the feasibility of using hydroxypropyl-β-cyclodextrin (HP-β-CD) to form a supramolecular inclusion complex with Naringin dihydrochalcone to improve its solubility. The inclusion complex was prepared by stirring an equal molar solution of Naringin dihydrochalcone and HP-β-CD at 30°C for 24 h, followed by freeze-drying.Results showed clearly the formation of a supramolecular complex in which the guest molecule, Naringin dihydrochalcone, was entrapped inside the cavity of the host, HP-β-CD. The close association between naringin DC and HP-β-CD resulted in changes in some of the characteristic spectral, phase transitional and morphological properties of Naringin dihydrochalcone. Furthermore, 1H-NMR analyses demonstrated that it was the B ring of Naringin dihydrochalcone that was inserted into the HP-β-CD cavity to form the supramolecular complex. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)