| Structure Identification: |
| Tetrahedron Letters,1991,32(14):1749–1752. | | Asymmetric aldol reactions using chiral boron reagents: Application to the synthesis of tirandamycin A[Reference: WebLink] |
METHODS AND RESULTS:
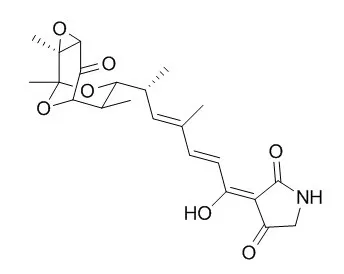
The bicyclic acetal 1, a key intermediate in previous synthetic studies on Tirandamycin A, has been prepared in enantiomerically pure form starting from the (R)-ethylketone 2 and the aldehyde 3. A reagent controlled aldol reaction using (-)-(Ipc)2BOTf selectively gave the 1,2-syn–2,4-syn adduct 4, which was subsequently converted into 1via the 1,3-anti diol 5. | | Journal of Medicinal Chemistry,1989 ,32 (5) :1062-9 | | Aromatic dienoyl tetramic acids. Novel antibacterial agents with activity against anaerobes and staphylococci[Reference: WebLink] | Streptolydigin (1) and Tirandamycin A (2) are typical members of the naturally occurring class of 3-dienoyl tetramic acids. These compounds, which possess potent antibacterial activity particularly against anaerobes, have been shown to inhibit bacterial RNA polymerase. In contrast, tenuazonic acid (5), which lacks a complex dioxabicyclononane moiety and diene chromophore present in 1 and 2, exhibits essentially no antimicrobial activity and has no effect on bacterial RNA polymerase, suggesting that one or both of these structural features may be critical for antibacterial activity.
METHODS AND RESULTS:
In this paper, we report on a novel series of synthetic dienoyl tetramic acids that lack a complex dioxabicyclononane unit. Several of these compounds, particularly 8T-W, exhibit potent antimicrobial activity against Gram-positive and Gram-negative anaerobes as well as staphylococci. We will discuss the structure-activity relationship for this series of compounds which, in contrast to their natural counterparts, do not inhibit significantly RNA polymerase. We will also discuss preliminary results on the biochemical and microbiological properties of this series of compounds, several of which moderately inhibit supercoiling by DNA gyrase isolated from E. coli H560, although this enzyme has not been established as their target in whole cells.
CONCLUSIONS:
Compound 8W, which is not cross-resistant with DNA gyrase subunit A or B inhibitors or tirandamycin, has also been demonstrated to be rapidly bactericidal. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)