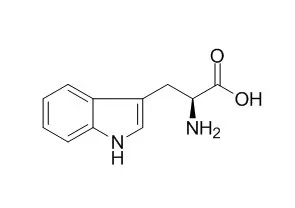
| Structure Identification: |
| Food Chem. 2015 Jan 1;166:596-602. | | Tryptophan-containing dipeptides are C-domain selective inhibitors of angiotensin converting enzyme.[Pubmed: 25053098] | Somatic angiotensin-converting enzyme (ACE) contains two active sites, the C- and N-domain, from which the C-domain is supposed to play a major role in blood pressure regulation and is therefore a promising pharmacological target to reduce blood pressure without side-effects.
METHODS AND RESULTS:
We report for the first time that Tryptophan-containing dipeptides such as Ile-Trp or Val-Trp, which were recently found in food protein hydrolysates, are selective and competitive inhibitors for the C-domain with a selectivity factor of 40 and 70, respectively. Structure-activity studies showed that an N-terminal aliphatic amino acid and a Tryptophan moiety in the P2' position are favourable structures for C-domain inhibition in dipeptides. In contrast, the lactotripeptides Ile-Pro-Pro and Val-Pro-Pro, which were widely used as ingredients for hypotensive food, showed a slight selectivity for the N-domain.
CONCLUSIONS:
Hence, Tryptophan containing dipeptides are interesting ingredients for functional foods as a natural prevention for hypertension with reduced side effects due to its selective inhibition of the C-domain. | | J Nucl Med. 2014 Oct;55(10):1605-10. | | Clinical significance of tryptophan metabolism in the nontumoral hemisphere in patients with malignant glioma.[Pubmed: 25189339] |
METHODS AND RESULTS:
Herein we tested whether the introduction of analogous, 'pseudo-pathogenic' Tryptophan mutations immediately after the bn cysteine (bn+1) in other cbEGF domains also caused protein folding/secretion challenges. We found that introduction of Tryptophan mutations into each of the four other F3 canonical cbEGF domains caused a significant reduction in protein secretion ranging from 2.7 to 56% of wild-type (WT) F3 levels. Surprisingly, an R185W mutation in the first canonical cbEGF domain of F3 yielded the highest amount of secretion among the F3 Tryptophan mutants, and its secretion defect could be rescued to near WT levels (95%) after growth temperature reduction. Interestingly, when similarly positioned Tryptophan mutations were introduced into any of the canonical cbEGF domains of the highly homologous protein, fibulin-5 (F5), there was no effect on secretion. In an attempt to make F3 tolerant of Tryptophan residues (like F5), we genetically engineered F3 to have a higher sequence homology with F5 by deleting three insert regions present in F3, but not F5. However, deletion of one or more of these regions did not have a beneficial effect on R345W F3 secretion.
CONCLUSIONS:
Overall, these results demonstrate that the introduction of Tryptophan residues at the bn+1 position does not universally disrupt cbEGF domain folding and secretion. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)