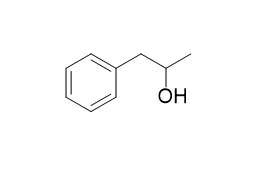
| Structure Identification: |
| J Phys Chem A. 2006 Dec 14;110(49):13188-94. | | Rotational spectra and conformational structures of 1-phenyl-2-propanol, methamphetamine, and 1-phenyl-2-propanone.[Pubmed: 17149832 ] |
METHODS AND RESULTS:
Microwave spectra have been recorded for 1-Phenyl-2-propanol, methamphetamine, and 1-phenyl-2-propanone from 11 to 24 GHz using a Fourier-transform microwave spectrometer.
Only one spectrum from a single conformational isomer was observed for each species. The rotational transitions in the spectrum of 1-phenyl-2-propanone were split into separate transitions arising from the A- and E-torsional levels of the methyl rotor. The fit of the E-state transitions to a "high-barrier" internal rotation Hamiltonian determines V3 = 238(1) cm-1 and rotor-axis angles of thetaa = 87.7(5) degrees, thetab = 50.0(5) degrees, and thetac = 40.0(5) degrees. Ab initio optimizations (MP2/6-31G**) and single-point calculations (MP2/6-311++G**) were used to model the structures of 1-Phenyl-2-propanol, methamphetamine, and 1-phenyl-2-propanone. The lowest energy conformations of these species were found to be stabilized by weak OH-pi, NH-pi, and CH-pi hydrogen-bonding interactions.
CONCLUSIONS:
Moments of inertia, derived from the model structures, were used to assign the spectra to the lowest energy conformation of each species. A series of MP2/6-31G* partial optimizations along the internal rotation pathway were used to estimate the barrier to methyl rotation to be 355 cm-1 for 1-phenyl-2-propanone. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)