A comparative study was made on the endogenous digoxin-like activity of sixteen mammalian-type lignan derivatives including enterolactone and enterodiol.
METHODS AND RESULTS:
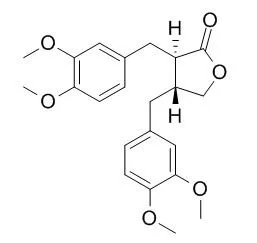
Cross-reactivity to antidigoxin antibody, inhibition of dog kidney Na+,K+-ATPase, and ouabain displacing activity against [3H]ouabain binding to human erythrocytes on the part of these derivatives were examined and compared in all cases with that of ouabain. meso-2,3-Dibenzylbutane-1,4-diol (meso-HA-1) was found to possess the most potent cross-reactivity to antidigoxin antibody. Several lignans, particularly HA-1 (either meso or dl), dl-2,3-bis(3-methoxybenzyl)butane-1,4-diol(HA-4), dl-2,3-bis(3,4-dimethoxybenzyl)butane-1,4-diol(HA-10), dl-2,3-bis(3,4-dimethoxybenzyl)-1,4-dimethoxybutane(HA-11), and trans-2,3-bis(3,4-dimethoxybenzyl)-gamma-butyrolactone(HA-14) were capable of inhibiting Na+,K+-ATPase activity with IC50 at a concentration less than 5 x 10(-4) M. Meso-HA-1, HA-11 and HA-14 also showed [3H]ouabain displacement activity with IC50 at a concentration of 10(-4)-10(-3) M. These determinations of activity were made at doses 100-1000 times those of ouabain.
CONCLUSIONS:
The synthetic lignans are considered to derive in vivo from plant material usually present in fibrous food. Based on the data obtained, some lignans may possibly be endogenous digitalis-like substances. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)