| In vitro: |
| Phytochemistry, 2007, 68(9):1312-1320. | | Activity-guided isolation of antiplasmodial dihydrochalcones and flavanones from Piper hostmannianum var. berbicense.[Reference: WebLink] |
METHODS AND RESULTS:
The bioassay-guided purification of an n-hexane extract from the leaves of Piper hostmannianum var. berbicense led to the isolation of four monoterpene or prenyl-substituted dihydrochalcones (1a, 1b, 2, 3) as well as the known compounds 2',6'-Dihydroxy 4'-methoxydihydrochalcone (4), linderatone (5), strobopinin (6), adunctin E (7) and (−)-methyllinderatin (8). Their structures were established on the basis of NMR and X-ray analysis.
CONCLUSIONS:
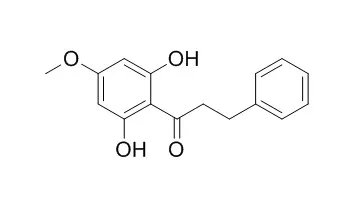
(−)-Methyllinderatin, linderatone and 2′,6′-dihydroxy-4′-methoxydihydrochalcone exhibited the most potent antiplasmodial activity with IC50 values of 5.64, 10.33 and 12.69 μM, respectively against both chloroquine-sensitive and resistant strains of Plasmodium falciparum (F32, FcB1). The activity of (−)-methyllinderatin was confirmed in vivo against Plasmodium vinckei petteri in mice (80% of reduction of parasitemia) at a dose of 20 mg/kg/day. | | Food Science and Technology International, Tokyo, 1997, 3(3):285-289. | | Antioxidative Constituents from the Aerial Part of Piper elongatum VAHL.[Reference: WebLink] |
METHODS AND RESULTS:
Six aromatic compounds, asebogenin (1), 2',6'-dihydroxy-4'-methoxydihydrochalcone (2), 3-geranyl-4-methoxy-benzoic acid (3), 3-geranyl-4-hydroxybenzoic acid (4), nervogenic acid (5) and 2,2-dimethyl-6-carboxyl-8-prenyl-chromene (6) were isolated from the methanol extract of the aerial part of Piper elongatum VAHL., whose leaves are used as a folk medicine in South America. The structures of 1-6 were elucidated by MS, 1H-NMR and 13C-NMR spectroscopies, and chemical evidence.
CONCLUSIONS:
Among these compounds, 1 showed stronger antioxidative activity than that of α-tocopherol, and 4 and 5 exhibited higher activity than that of t-butyl-4-hydroxyanisole (BHA) using the ferric thiocyanate method. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)