Type 2 diabetes (T2D) constituted 90% of the global 387 million diabetes cases in 2014. The enzyme protein-tyrosine phosphatase 1B (PTP1B) has been recognized as a therapeutic target for treatment of T2D and its adverse complications.
METHODS AND RESULTS:
With the aim of accelerating the investigation of complex natural sources, such as crude plant extracts, for potential PTP1B inhibitors, we have developed a bio-analytical platform combining high-resolution PTP1B inhibition profiling and high-performance liquid chromatography-high-resolution mass spectrometry-solid-phase extraction-nuclear magnetic resonance spectroscopy, i.e., HR-bioassay/HPLC-HRMS-SPE-NMR. Human recombinant PTP1B enzyme was used for the microplate-based PTP1B inhibition assay, which was optimized for pH and substrate concentration to be compatible with rate measurements within the 10 min incubation time. Subsequently, analytical-scale HPLC-based microfractionation followed by colorimetric microplate-based PTP1B bioassaying enabled construction of a high-resolution inhibition profile corresponding to the HPLC profile. The high-resolution PTP1B inhibition profiling was validated using an artificial mixture of known PTP1B inhibitors and non-inhibiting compounds as negative controls. Finally, a proof-of-concept study with a real sample was performed using crude ethyl acetate extract of the phytochemically hitherto unexplored plant Eremophila lucida.
CONCLUSIONS:
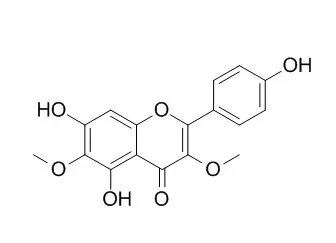
This led to the identification of the first viscidane type diterpene, i.e., 5-hydroxyviscida-3,14-dien-20-oic acid (9) as PTP1B inhibitor with an IC50 value of 42.0 ± 5.9 μM. In addition, a series of flavonoids, i.e., luteolin (1), dinatin (3a), tricin (3b), 3,6-Dimethoxyapigenin (4), jaceidin (5), and cirsimaritin (6) as well as a cembrene diterpene, (3Z, 7E, 11Z)-15-hydroxycembra-3,7,11-trien-19-oic acid (8), were also identified for the first time from E. lucida. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)