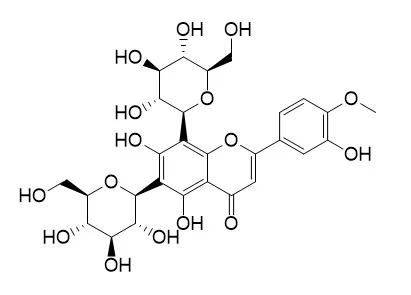
| Structure Identification: |
| J Agric Food Chem . 2013 Feb 27;61(8):1686-93 | | HPLC-PDA-MS and NMR characterization of a hydroalcoholic extract of Citrus aurantium L. var. amara peel with antiedematogenic activity[Pubmed: 22957519] | | The phytochemical profile of a hydroalcoholic extract of Citrus aurantium var. amara L. peel, used as herbal medicine, was characterized by HPLC-PDA-MS. Two di-C-glycosyl flavones (vincenin II and diosmetin 6,8-di-C-glucoside), a series of flavones (luteolin 7-O-neohesperidoside, rhoifolin, and neodiosmin), and flavanone (neoeriocitrin, naringin, and neohesperidin) 7-O-neohesperidosides and two methoxyflavones (nobiletin and tangeretin), commonly present in Citrus, were identified. Furthermore, brutieridin and melitidin, two 3-hydroxy-3-methylglutaryl flavanone glycosides, were also characterized along with rhoifolin 4'-glucoside and three coumarins (8,3'-β-D-glucopyranosyloxy-2'-hydroxy-3'-methylbutyl-7-methoxycoumarin, merazin hydrate, and isomerazin). A preparative isolation procedure followed by NMR spectroscopy confirmed the proposed structures of the major flavonoids and identified the coumarins. The phenolic content was found to be 14.8 mg mL(-1), and naringin and neohesperidin were the compounds present in the highest concentration (3.6 and 2.6 mg mL(-1)). The extract of C. aurantium peel inhibited significantly (p < 0.05) both histamine- and dextran-induced edema in rats in a concentration-dependent manner (IC(50) = 119.6 and 118.3 mg kg(-1), respectively), providing evidence for the therapeutic use of C. aurantium var. amara peel. | | Biosci Biotechnol Biochem. 2007 Aug;71(8):1911-1199. | | Isolation of antioxidative phenolic glucosides from lemon juice and their suppressive effect on the expression of blood adhesion molecules[Pubmed: 17690486] | | Phenolic glucosides having radical scavenging activity were examined from the fraction eluted with 20% methanol on Amberlite XAD-2 resin applied to lemon (Citrus limon) juice by using reversed phase chromatography. Four phenolic glucosides were identified as 1-feruloyl-beta-D-glucopyranoside, 1-sinapoyl-beta-D-glucopyranoside, 6,8-di-C-glucosylapigenin and 6,8-di-C-glucosyldiosmetin by (1)H-NMR, (13)C-NMR, and MS analyses. They exhibited radical scavenging activity for 1,1-diphenyl-2-picrylhydrazyl (DPPH) and superoxide, although the activity was low in comparison with eriocitrin, a potent antioxidant in lemon fruit, and the eriodictyol of its aglycone. The phenolic compounds in lemon juice were examined for their suppressive effect on the expression of blood adhesion molecules by measuring the expression of intercellular adhesion molecule-1 (ICAM-1) in human umbilical vein endothelial cells (HUVECs) induced by necrosis factor-alpha (TNF-alpha). 6,8-Di-C-glucosylapigenin, apigenin, and diosmentin of the flavones were found to significantly suppress the expression of ICAM-1 at 10 muM (P<0.05). The phenolic glucosides isolated in this study were contained in comparative abundance in daidai (Citrus aurantium) and niihime (Citrus unshiu x Citrus tachibana) among the sour citrus juices. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)