| Kinase Assay: |
| Toxicology & applied pharmacology, 2011, 254(3):288-298. | | 5-Methoxyflavanone induces cell cycle arrest at the G2/M phase, apoptosis and autophagy in HCT116 human colon cancer cells.[Reference: WebLink] | Natural flavonoids have diverse pharmacological activities, including anti-oxidative, anti-inflammatory, and anti-cancer activities.
METHODS AND RESULTS:
In this study, we investigated the molecular mechanism underlying the action of 5-Methoxyflavanone (5-MF) which has a strong bioavailability and metabolic stability. Our results show that 5-MF inhibited the growth and clonogenicity of HCT116 human colon cancer cells, and that it activated DNA damage responses, as revealed by the accumulation of p53 and the phosphorylation of DNA damage-sensitive proteins, including ataxia–telangiectasia mutated (ATM) at Ser1981, checkpoint kinase 2 (Chk2) at Thr68, and histone H2AX at Ser139. 5-MF-induced DNA damage was confirmed in a comet tail assay. We also found that 5-MF increased the cleavage of caspase-2 and -7, leading to the induction of apoptosis. Pretreatment with the ATM inhibitor KU55933 enhanced 5-MF-induced γ-H2AX formation and caspase-7 cleavage. HCT116 cells lacking p53 (p53−/−) or p21 (p21−/−) exhibited increased sensitivity to 5-MF compared to wild-type cells. 5-MF further induced autophagy via an ERK signaling pathway. Blockage of autophagy with the MEK inhibitor U0126 potentiated 5-MF-induced γ-H2AX formation and caspase-2 activation.
CONCLUSIONS:
These results suggest that a caspase-2 cascade mediates 5-MF-induced anti-tumor activity, while an ATM/Chk2/p53/p21 checkpoint pathway and ERK-mediated autophagy act as a survival program to block caspase-2-mediated apoptosis induced by 5-MF. |
|
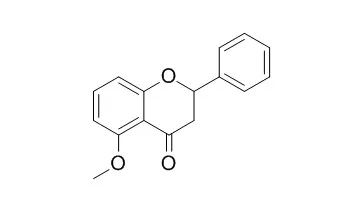
| Structure Identification: |
| Natural Product Research, 2009, 23(13):1231-1239. | | Microbial metabolism. Part 10: Metabolites of 7,8-dimethoxyflavone and 5-methoxyflavone.[Reference: WebLink] |
METHODS AND RESULTS:
Microbial transformation of 7,8-dimethoxyflavone (1) by Mucor ramannianus produced five metabolites: 7,8-dimethoxy-4′-hydroxyflavone (2), 3′,4′-dihydroxy-7,8-dimethoxyflavone (3), 7,3′-dihydroxy-8-methoxyflavone (4), 7,4′-dihydroxy-8-methoxyflavone (5) and 8-methoxy-7,3′,4′-trihydroxyflavone (6). It was, however, completely converted to a single metabolite, 7-hydroxy-8-methoxyflavone (7) by Aspergillus flavus. 5-Methoxyflavone (8), when fermented with Beauveria bassiana, gave a single product, 5-Methoxyflavanone (9). Conversion of 8 with Aspergillus alliaceus yielded the metabolite 4′-hydroxy-5-methoxyflavone (10). The structures of the compounds 2–7, 9 and 10 were established by spectroscopic methods. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)