| Kinase Assay: |
| Food Funct . 2019 May 22;10(5):2881-2887. | | Dihydrochalcone-derived polyphenols from tea crab apple (Malus hupehensis) and their inhibitory effects on α-glucosidase in vitro[Pubmed: 31070208] | | Three dihydrochalcone-derived polyphenols, huperolides A-C (1-3), along with thirteen known compounds (4-16) were isolated from the leaves of Malus hupehensis, the well-known tea crab apple in China. Their chemical structures were elucidated by extensive spectroscopic analysis including NMR (HSQC, HMBC, 1H-1H COSY and ROESY), HRMS and CD spectra. Huperolide A is a polyphenol with a new type of carbon skeleton, while huperolides B and C are a couple of atropisomers, which were isolated from natural sources for the first time. The antihyperglycemic effects of the isolated compounds were evaluated based on assaying their inhibitory activities against α-glucosidase. As a result, phlorizin (4), 3-hydroxyphloridzin (5), 3-O-coumaroylquinic acid (12) and β-hydroxypropiovanillone (15) showed significant concentration-dependent inhibitory effects on α-glucosidase. Therefore, those compounds might be responsible for the antihyperglycemic effect of this herb, and are the most promising compounds to lead discovery of drugs against diabetes. |
|
| Structure Identification: |
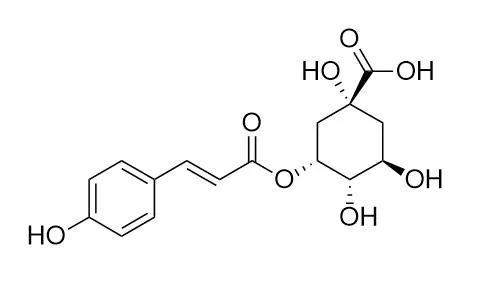
| Fitoterapia . 2014 Dec;99:1-6. | | Caffeoylquinic acid derivatives isolated from the aerial parts of Gynura divaricata and their yeast α-glucosidase and PTP1B inhibitory activity[Pubmed: 25172103] | | The phytochemical investigation of natural products of Gynura divaricata led to the isolation of eleven caffeoylquinic acid derivatives. They were characterized by spectrometric methods as 5-O-caffeoylquinic acid (1), 5-O-p-coumaroylquinic acid (2), 5-O-feruloylquinic acid (3), methyl 5-O-caffeoylquinate (4), 3,4-dicaffeoylquinic acid (5), 3,5-dicaffeoylquinic acid (6), 4,5-dicaffeoylquinic acid (7), methyl 3,4-dicaffeoylquinate (8), methyl 3,5-dicaffeoylquinate (9), methyl 4,5-dicaffeoylquinate (10) and ethyl 4,5-dicaffeoylquinate (11). The individual compounds were screened for the inhibition of yeast α-glucosidase and Protein Tyrosine Phosphatase 1B (PTP1B) using in vitro assays. Among the isolated compounds, 3,4-dicaffeoylquinic acid (5), 4,5-dicaffeoylquinic acid (7), methyl 3,4-dicaffeoylquinate (8) and methyl 4,5-dicaffeoylquinate (10) exhibited significant inhibitory activities against α-glucosidase. In addition, 5-O-p-coumaroylquinic acid (2), 3,5-dicaffeoylquinic acid (6) and 4,5-dicaffeoylquinic acid (7) had considerable inhibitory effect against PTP1B. Based on these findings, the caffeoylquinic acid derivatives were deduced to be potentially responsible for the anti-diabetic activity of G. divaricata. The preliminary structure-activity relationship study suggests that the number and positioning of caffeoyl groups in the quinic acid derivatives are important for both α-glucosidase and PTP1B inhibitory potency. Moreover, the corresponding methyl esters of some dicaffeoylquinic acids have enhanced inhibitory activity against yeast α-glucosidase. | | Food Funct . 2019 May 22;10(5):2881-2887. | | Dihydrochalcone-derived polyphenols from tea crab apple (Malus hupehensis) and their inhibitory effects on α-glucosidase in vitro[Pubmed: 31070208] | | Three dihydrochalcone-derived polyphenols, huperolides A-C (1-3), along with thirteen known compounds (4-16) were isolated from the leaves of Malus hupehensis, the well-known tea crab apple in China. Their chemical structures were elucidated by extensive spectroscopic analysis including NMR (HSQC, HMBC, 1H-1H COSY and ROESY), HRMS and CD spectra. Huperolide A is a polyphenol with a new type of carbon skeleton, while huperolides B and C are a couple of atropisomers, which were isolated from natural sources for the first time. The antihyperglycemic effects of the isolated compounds were evaluated based on assaying their inhibitory activities against α-glucosidase. As a result, phlorizin (4), 3-hydroxyphloridzin (5), 3-O-coumaroylquinic acid (12) and β-hydroxypropiovanillone (15) showed significant concentration-dependent inhibitory effects on α-glucosidase. Therefore, those compounds might be responsible for the antihyperglycemic effect of this herb, and are the most promising compounds to lead discovery of drugs against diabetes. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)